Clobetasol Eye Drops: Package Insert / Prescribing Info
Package insert / product label
Generic name: clobetasol propionate
Dosage form: ophthalmic suspension
Medically reviewed by Drugs.com. Last updated on Apr 22, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
BYQLOVI,
for topical ophthalmic use
Initial U.S. Approval: 1985
Indications and Usage for Clobetasol Eye Drops
BYQLOVI is a corticosteroid indicated for the treatment of post-operative inflammation and pain following ocular surgery. ( 1)
Clobetasol Eye Drops Dosage and Administration
Dosage Forms and Strengths
Ophthalmic suspension containing clobetasol propionate 0.05%. ( 3)
Contraindications
BYQLOVI is contraindicated in most active viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. ( 4)
Warnings and Precautions
- Intraocular Pressure (IOP) Increase: Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. If this product is used for 10 days or longer, IOP should be monitored. ( 5.1)
- Cataracts: Prolonged use of corticosteroids may result in posterior subcapsular cataract formation. ( 5.2)
- Delayed Healing: The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. ( 5.3)
- Corneal and Scleral Melting: In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. The initial prescription and renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and where appropriate, fluorescein staining. ( 5.4)
- Bacterial Infections: Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be reevaluated. ( 5.5)
- Viral Infections: Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). ( 5.6)
- Fungal Infections: Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application. Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use. Fungal culture should be taken when appropriate. ( 5.7)
Adverse Reactions/Side Effects
Ocular adverse reactions occurring in ≥ 1% of subjects in clinical studies who received BYQLOVI included eye inflammation (2%), corneal edema (2%), anterior chamber inflammation (2%), cystoid macular edema (2%), intraocular pressure elevation (1%), photophobia (1%) and vitreous detachment (1%). Many of these reactions may have been the consequence of the surgical procedure ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Eyenovia, Inc. at 1-833-393-6684 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2025
Full Prescribing Information
1. Indications and Usage for Clobetasol Eye Drops
BYQLOVI is indicated for the treatment of post-operative inflammation and pain following ocular surgery.
2. Clobetasol Eye Drops Dosage and Administration
3. Dosage Forms and Strengths
Ophthalmic suspension containing clobetasol propionate 0.05% (0.5 mg/mL).
4. Contraindications
BYQLOVI is contraindicated in most active viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
5. Warnings and Precautions
5.1 Intraocular Pressure (IOP) Increase
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. If BYQLOVI is used for 10 days or longer, IOP should be monitored.
5.2 Cataracts
Prolonged use of corticosteroids may result in posterior subcapsular cataract formation.
5.3 Delayed Healing
The use of corticosteroids after cataract surgery may delay healing and increase the incidence of bleb formation.
5.4 Corneal and Scleral Melting
In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical corticosteroids. The initial prescription and renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and where appropriate, fluorescein staining .
5.5 Bacterial Infections
Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be reevaluated.
5.6 Viral Infections
Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
5.7 Fungal Infections
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal culture should be taken when appropriate.
6. Adverse Reactions/Side Effects
The following serious reactions are found elsewhere in the labeling:
- Intraocular Pressure (IOP) Increase [see Warnings and Precautions (5.1)]
- Posterior Subcapsular Cataract Formation [see Warnings and Precautions (5.2)]
- Delayed Healing [see Warnings and Precautions (5.3)]
- Corneal and Scleral Melting [see Warnings and Precautions (5.4)]
- Bacterial Infections [see Warnings and Precautions (5.5)]
- Viral Infections [see Warnings and Precautions (5.6)]
- Fungal Infections [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ocular adverse reactions occurring in ≥ 1% of subjects in clinical studies who received BYQLOVI included eye inflammation (2%), corneal edema (2%), anterior chamber inflammation (2%), cystoid macular edema (2%), intraocular pressure elevation (1%), photophobia (1%) and vitreous detachment (1%). Many of these reactions may have been the consequence of the surgical procedure.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled clinical studies of BYQLOVI administration in pregnant women to inform drug-associated risks. Plasma concentrations of clobetasol propionate were minimal following topical ophthalmic administration of BYQLOVI [see Clinical Pharmacology (12.3)] . However, corticosteroids, including clobetasol propionate have been shown to be teratogenic and fetotoxic in laboratory animals when administered systemically at relatively low dosage levels (see Data) .
BYQLOVI should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In embryofetal development studies in mice, clobetasol propionate was fetotoxic at the highest subcutaneous dose tested (1 mg/kg) and teratogenic at all subcutaneous dose levels tested down to 0.03 mg/kg. Abnormalities observed included cleft palate and skeletal abnormalities. These doses are approximately 98 times and 3 times, respectively, the recommended human ophthalmic dose of BYQLOVI, estimated based on body surface area and assuming 100% systemic absorption.
In embryofetal development studies in rabbits, clobetasol propionate was teratogenic at subcutaneous doses of 3 and 10 µg/kg. Abnormalities seen included cleft palate, cranioschisis, and other skeletal abnormalities. These doses are approximately 1.2 times and 3.9 times, respectively, the recommended human ophthalmic dose of BYQLOVI, estimated based on body surface area and assuming 100% systemic absorption.
8.2 Lactation
Risk Summary
There is no information regarding the presence of clobetasol propionate in human milk, the effects on the breastfed infant, or the effects on milk production.
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. However, systemic levels of clobetasol propionate following topical ocular administration are minimal [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of clobetasol propionate would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BYQLOVI, and any potential adverse effects on the breastfed infant from BYQLOVI.
11. Clobetasol Eye Drops Description
BYQLOVI (clobetasol propionate ophthalmic suspension) 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, that has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
Chemically, clobetasol propionate is 21-chloro-9-fluoro-11β,17-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-propionate and it has the following structural formula:
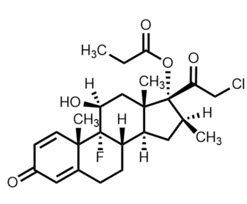
Clobetasol propionate has the empirical formula C 25H 32CIFO 5and a molecular weight of 467.
BYQLOVI contains a sterile, anti-inflammatory corticosteroid for topical ophthalmic use.
Each mL of BYQLOVI contains:
ACTIVE: clobetasol propionate 0.5 mg (0.05%)
INACTIVES: sodium chloride, hydrogenated soybean lecithin, citric acid, glycerin, poloxamer 407, polyvinyl alcohol, boric acid, edetate disodium dihydrate, methylcellulose, trisodium citrate, and water for injection
PRESERVATIVE: benzalkonium chloride 0.0036%.
12. Clobetasol Eye Drops - Clinical Pharmacology
12.1 Mechanism of Action
Like other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A 2inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2.
12.2 Pharmacodynamics
In patients treated with BYQLOVI twice daily for 21 days, the mean (SD) changes from baseline in cortisol concentrations, -18.1 (126.2) nmol/L and +5.1 (129.8) nmol/L for the BYQLOVI and matching vehicle arms, respectively, were not statistically significant, considering the variability observed in the cortisol concentrations.
12.3 Pharmacokinetics
After the first and second (12 hours apart) ocular instillations of BYQLOVI in healthy adults (n=12), peak plasma clobetasol propionate concentrations (C max) were below the lower limit of quantitation (LLOQ, 0.04 ng/mL) in 13 out of 22 PK profiles and ranged from 0.040 to 0.182 ng/mL in the other 9 profiles. Time to peak concentration (T max) was observed between 0.5 - 1 hour post-dose. Clobetasol propionate concentrations declined to lower than LLOQ after 4 to 5 hours post-dose.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies have not been performed with BYQLOVI to evaluate the carcinogenic potential of clobetasol propionate.
Mutagenesis
Clobetasol propionate was not mutagenic in 3 different test systems: the Ames test, the Saccharomyces cerevisiae gene conversion assay, and the E. coli B WP2 fluctuation test.
Impairment of Fertility
Fertility studies in the rat following subcutaneous administration at dosage levels up to 50 µg/kg/day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose. This dose is approximately 10 times the recommended human ophthalmic dose of BYQLOVI (0.83 μg/kg/day), estimated based on body surface area and assuming 100% systemic absorption. Note that systemic levels of clobetasol propionate following topical ocular administration are minimal [see Clinical Pharmacology (12.3)] .
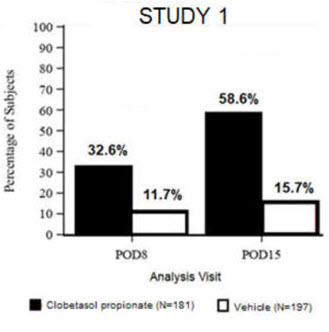
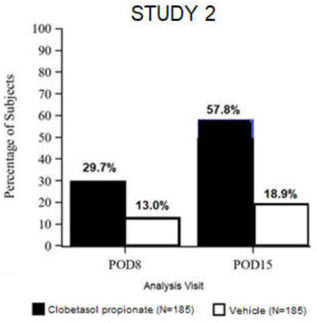
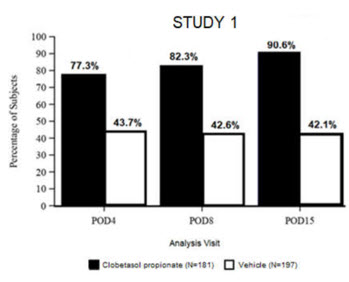
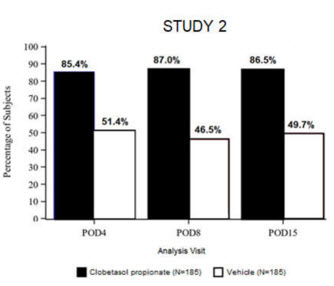
14. Clinical Studies
Clinical efficacy was evaluated in 2 multi-center, randomized, double-masked, vehicle-controlled trials in which patients had ≥10 cells in the anterior chamber after cataract surgery were assigned to BYQLOVI 0.05% (N=366) or vehicle (N=382) ( NCT04739709(Study 1) and NCT04810962(Study 2)). One drop of Clobetasol Propionate Ophthalmic Suspension 0.05% or vehicle was self-administered twice a day for 14 days, beginning on the day after surgery. Complete resolution of inflammation (an anterior chamber cell count of 0 maintained through Day 15 without rescue medication) and complete resolution of pain (a patient-reported pain grade of 0 maintained through Day 15 without rescue medication) were assessed at post-operative day (POD) 4, 8, and 15. (Study 1) and NCT04810962(Study 2)). One drop of BYQLOVI 0.05% or vehicle was self-administered twice a day for 14 days, beginning on the day after surgery. Complete resolution of inflammation (an anterior chamber cell count of 0 maintained through Day 15 without rescue medication) and complete resolution of pain (a patient-reported pain grade of 0 maintained through Day 15 without rescue medication) were assessed at post-operative day (POD) 4, 8, and 15.
The co-primary efficacy endpoints were the proportion of subjects with Anterior Chamber Cell (ACC) count = 0 (ACC grade = 0) at POD8 maintained through POD15, and the proportion of subjects with Ocular Pain Grade = 0 at POD4 maintained through POD15. In the intent-to-treat analysis, both co-primary efficacy endpoints were statistically significantly better in BYQLOVI 0.05%-treated patients compared to vehicle-treated patients (p<0.01). The clinical trial efficacy results from both studies are provided below.
| Figure 1: Percent of Patients with Anterior Chamber Cell Count = 0 at Post-Operative Days 8 and 15 | |
|---|---|
|
|
|
| Figure 2: Percent of Patients with Complete Resolution of Pain at Post-Operative Days 4, 8, and 15 | |
|---|---|
|
|
|
16. How is Clobetasol Eye Drops supplied
16.1 How Supplied
BYQLOVI (clobetasol propionate ophthalmic suspension) 0.05% (0.5 mg/mL) is a sterile ophthalmic suspension. It is supplied in a multi-dose white low-density polyethylene plastic 5 mL eye-dropper bottle with a low-density polyethylene white tip and a high-density polyethylene pink cap with a tamper-proof ring at the bottom of the cap.
3.5 mL in a 5 mL bottle (NDC 81046-0319-1)
17. Patient Counseling Information
Administration with Other Eye Drops
Advise patients to wait at least 5 minutes between instillation of BYQLOVI and other eye drops if using other eye drops in addition to BYQLOVI.
Risk of Contamination
Advise patients to wash their hands well before each use. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the ophthalmic suspension.
| CLOBETASOL PROPIONATE
clobetasol propionate suspension/ drops |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Eyenovia, Inc. (081032969) |