Claritin-D 24 Hour: Package Insert / Prescribing Info
Package insert / product label
Generic name: loratadine and pseudoephedrine sulfate
Dosage form: tablet, extended release
Drug class: Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Dec 12, 2024.
Indications and Usage for Claritin-D 24 Hour
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- itchy, watery eyes
- runny nose
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Claritin-D 24 Hour Dosage and Administration
- do not divide, crush, chew or dissolve the tablet
| adults and children 12 years and over | 1 tablet daily with a full glass of water; not more than 1 tablet in 24 hours |
| children under 12 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Storage and Handling
- each tablet contains: calcium 25 mg
- safety sealed: do not use if the individual blister unit imprinted with Claritin-D ® 24 hour is open or torn
- store between 20° to 25°C (68° to 77°F)
- protect from light and store in a dry place
Inactive ingredients
carnauba wax, dibasic calcium phosphate dihydrate, ethylcellulose, hydroxypropyl cellulose, hypromellose, magnesium stearate, pharmaceutical ink, polyethylene glycol, povidone, silicon dioxide, sucrose, titanium dioxide
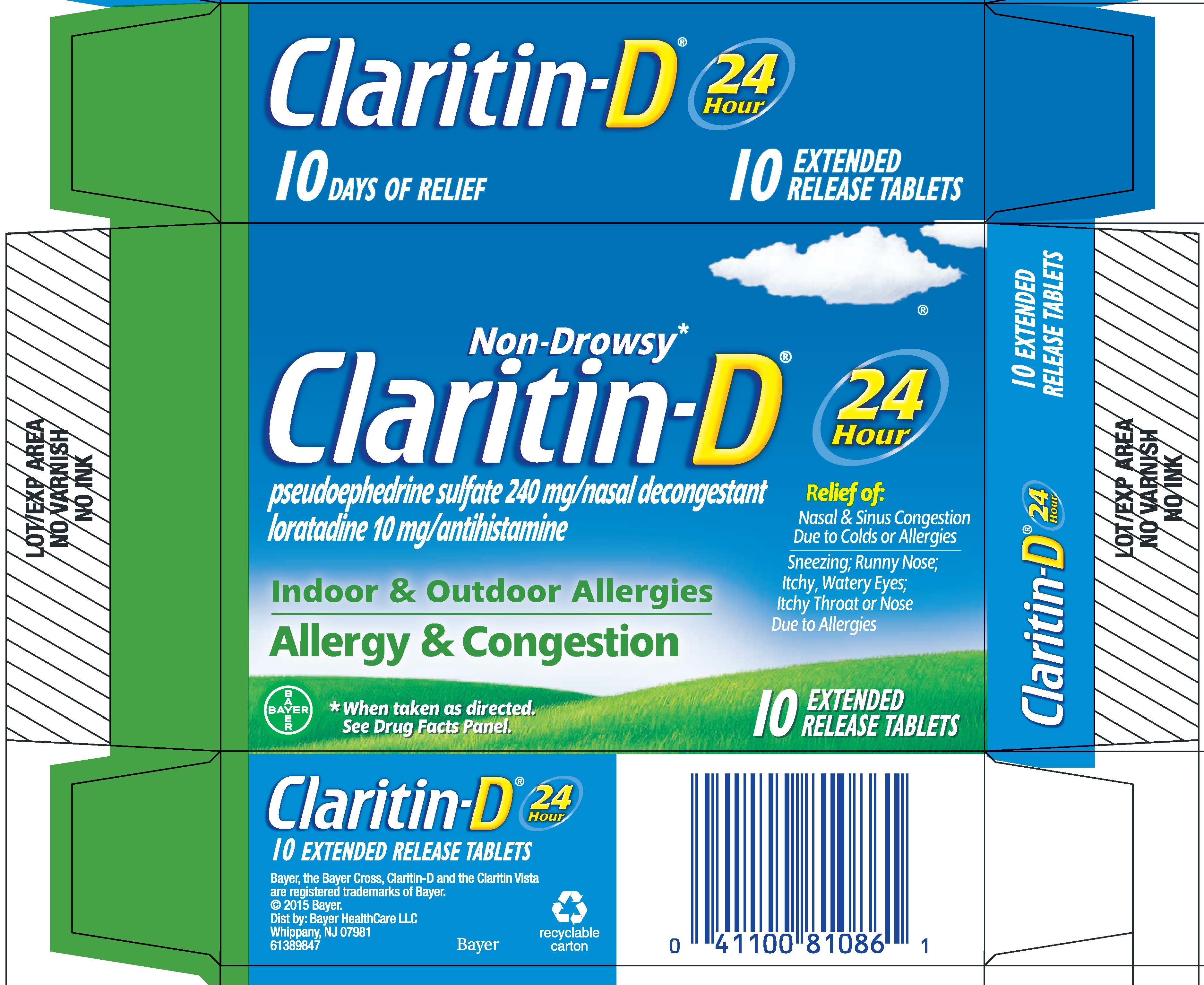
PRINCIPAL DISPLAY PANEL - 10 Tablet Blister Pack Carton
NDC 11523-4332-1
Non-Drowsy*
Claritin-D ®
pseudoephedrine sulfate 240 mg/nasal decongestant
loratadine 10 mg/antihistamine
Indoor & Outdoor Allergies
Allergy & Congestion
24
Hour
Relief of:
Nasal & Sinus Congestion
Due to Colds or Allergies
Sneezing; Runny Nose;
Itchy, Watery Eyes;
Itchy Throat or Nose
Due to Allergies
* When taken as directed. See Drug Facts Panel.
5
EXTENDED
RELEASE TABLETS

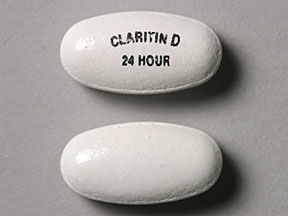
| CLARITIN-D
24 HOUR
loratadine and pseudoephedrine sulfate tablet, extended release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
More about Claritin-D 24 Hour (loratadine / pseudoephedrine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (12)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: upper respiratory combinations
- En español
Patient resources
Professional resources
Other brands
Alavert D-12 Hour Allergy and Sinus