Chromagen: Package Insert / Prescribing Info
Package insert / product label
Generic name: iron supplement
Dosage form: capsule
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Apr 14, 2025.
On This Page
WARNING:Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
DESCRIPTION: Chromagen for oral administration is an iron supplement softgel that is pearlescent dark blue and embossed with CH75 on one side.
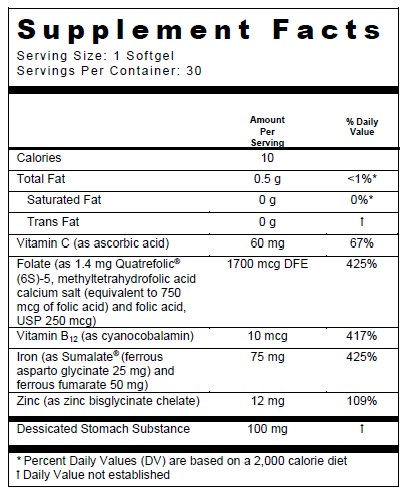
OTHER INGREDIENTS: Beeswax, soybean oil and soy lecithin. Gelatin capsule (bovine gelatin, Candurin® Blue Shimmer (mica-based pearlescent pigment), caramel coloring, carob, glycerin, FD&C Blue #1, purified water).
INDICATIONS: Chromagen™ is a multivitamin/multimineral dietary supplement indicated for use in improving the nutritional status of patients with iron deficiency.
CONTRAINDICATIONS: Chromagen™ is contraindicated in patients with a known hypersensitivity to any of the ingredients.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B 12is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS:Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
WARNING:Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
HOW SUPPLIED: Bottles of 30 softgels (75854-320-30). The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
STORAGE:Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OFTHE REACH OF CHILDREN.
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
MANUFACTURED FOR:
Avion Pharmaceuticals, LLC
Alpharetta, GA 30005 1-888-61-AVION
L-0203 Rev. 0325-02
Quatrefolic® is a registered trademark of Gnosis, SpA. Covered by one or more claims of U.S. Patent # 7,947,662
CAS# 1181972-37-1
Sumalate® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 6,716,814,8,007,846 and 8,425, 956.
| CHROMAGEN
ferrous asparto glycinate, ferrous fumarate, ascorbic acid, folic acid, cyanocobalamin, zinc, and intrinsic factor capsule |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Avion Pharmaceuticals, LLC (040348516) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avion Pharmaceuticals, LLC | 040348516 | manufacture(75854-320) | |
More about Chromagen (multivitamin with iron)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: iron products
Professional resources
Other brands
Dialyvite, Integra Plus, NovaFerrum, Ferrex 150 Forte, ... +11 more