Calcium Folinate Injection: Package Insert / Prescribing Info
Package insert / product label
Dosage form: injection, solution
Drug class: Antidotes
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
URGENT: LEUCOVORIN UPDATE
June 2011
Dear Healthcare Professional:
Due to the current critical shortage of Leucovorin Calcium Injection in the United States market, the FDA has asked Teva to help increase the availability of this product. The following letter is being sent to you at FDA’s request.
Teva is working with the FDA to initiate a temporary importation of the product from our European counterparts. The European product, known as Calcium Folinate Solution for Injection, has not previously been reviewed or approved by the FDA, but is of the same qualitative formulation as Leucovorin Calcium for Injection previously approved for marketing in the U.S. when the latter is reconstituted with water. Calcium Folinate Solution for Injection is manufactured in accord with European Good Manufacturing Practice regulations at our site in Godollo, Hungary and marketed through our Teva UK affiliate.
At this time, no other entity except Teva has been given regulatory enforcement discretion to import or distribute Calcium Folinate Solution for Injection. Any sales of Calcium Folinate Solution for Injection from any entity other than Teva will be considered in violation of the Federal Food, Drug and Cosmetic Act and may be subject to enforcement action by the FDA.
Effective immediately, and pursuant to the FDA’s authorization, Teva will offer Leucovorin Calcium (Calcium Folinate) in the following versions:
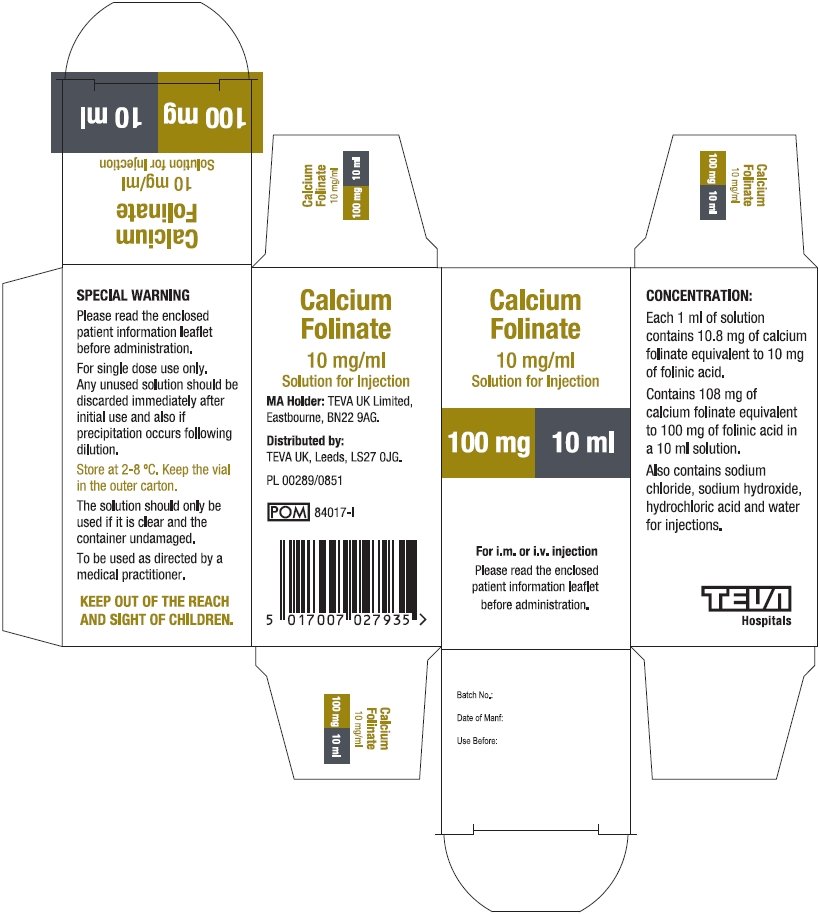
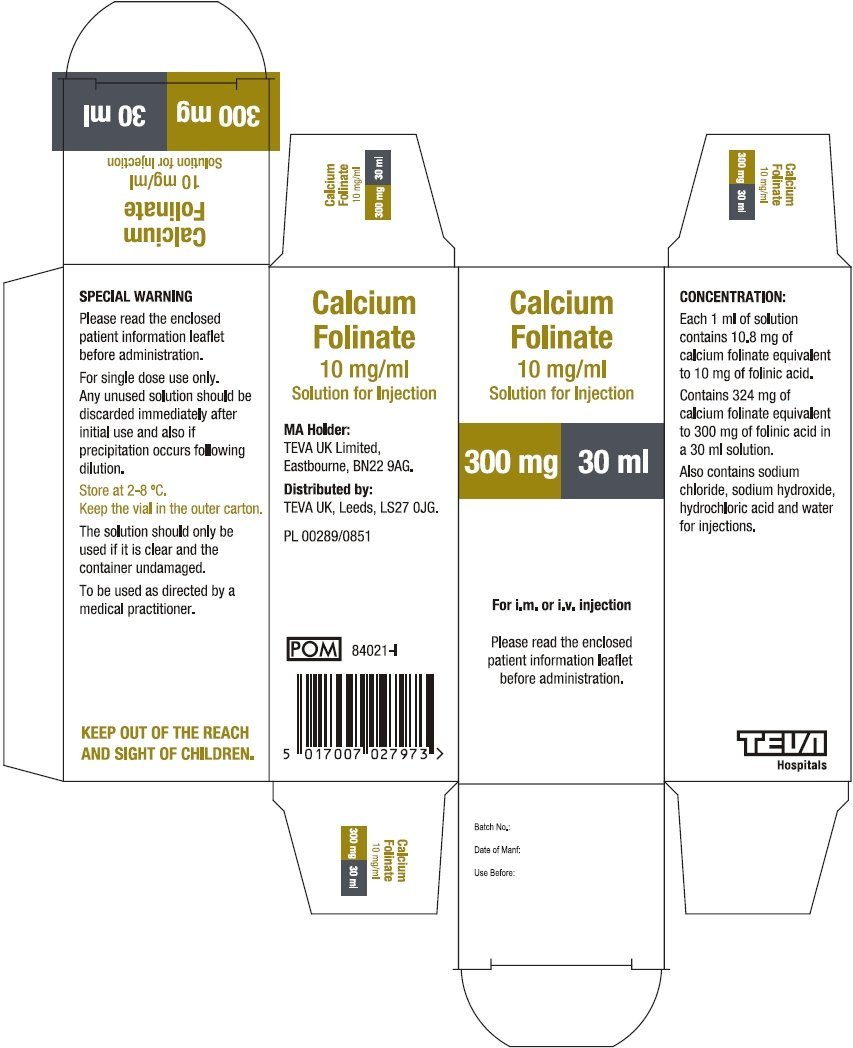
10 mg folinic acid/mL in vial sizes of 10 and 30 mL
Calcium Folinate Solution for Injection contains the same active ingredient in the same concentration as Leucovorin Calcium for Injection when the latter is reconstituted with water. The naming convention for the active ingredient varies. The barcode used on Calcium Folinate Solution for Injection may not be appropriately recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is used. Alternative procedures should be implemented to assure that the correct drug product is being dispensed and administered to individual patients.
For questions regarding Calcium Folinate Solution for Injection in the United States, please contact Teva Medical Affairs at (888) 838-2872 or (800) 227-7522 between the hours of 8 am and 5 pm EST. This communication and updated product information is available on the Teva website at www.tevausa.com as well as on the FDA Drug Shortage website at www.fda.gov/Drugs/DrugSafety/DrugShortages.
The product comparison table below highlights the minor differences between Leucovorin Calcium for Injection and Calcium Folinate Solution for Injection.
| Teva U. S. Leucovorin Calcium for Injection | Teva U.K. Calcium Folinate Solution for Injection | |
| Active ingredient | Leucovorin calcium | Calcium folinate (aka leucovorin calcium) |
| Potency | 10mg/ mL after reconstitution | 10 mg/ mL |
| Inactive ingredients |
Sodium chloride Hydrochloric acid Sodium hydroxide Product can be reconstituted with Water for Injection |
Sodium chloride Hydrochloric acid Sodium hydroxide Water for injection |
| Indications |
Leucovorin calcium rescue is indicated:
5-day treatments repeated at 4-week intervals:
or
Calcium folinate is indicated:
5-day treatments repeated at 4-week intervals:
or
|
| Teva U. S. Leucovorin Calcium for Injection | Teva U.K. Calcium Folinate Solution for Injection | |
| Indications (continued) |
Regimens in other US labels but NOT in US leucovorin label: Leucovorin 200 mg/m2 followed by 400 mg/m2 5-FU bolus and 22-hour infusion of 5-FU (600 mg/m2) for on days 1, 2, 15, 16, 29, 30 plus CAMPTOSAR 180 mg/m2 IV over 90 min, on days 1, 15, 29 Day 1: ELOXATIN 85 mg/m2 IV infusion and Leucovorin 200 mg/m2 followed by 400 mg/m2 5-FU bolus and 22-hour infusion of 5-FU (600 mg/m2) for 2 consecutive days every 2 weeks on days 1 and 2 Day 2:. Leucovorin 200 mg/m2 followed by 400 mg/m2 5-FU bolus and 22-hour infusion of 5-FU (600 mg/m2) for 2 consecutive days every 2 weeks on days 1 and 2 Leucovorin 20 mg/m2 IV as IV infusion plus 500 mg/m2 5-FU as IV bolus injection on days 1, 8, 15, 22 plus CAMPTOSAR 125 mg/m² I V over 90 min, on days 1, 8, 15, 22 Leucovorin should not be mixed in the same infusion as 5-fluorouracil because a precipitate may form. |
Regimens NOT in any US label: Bimonthly regimen: Leucovorin 200 mg/m2 followed by 400 mg/m2 5-FU bolus and 22-hour infusion of 5-FU (600 mg/m2) for 2 consecutive days every 2 weeks on days 1 and 2 Weekly regimen: Leucovorin 20 mg/m2 IV injection or as IV infusion plus 500 mg/m2 5-FU as IV bolus injection in the middle or at the end of leucovorin |
| Route of administration | Intravenously (injection or infusion) or intramuscularly after reconstitution | Intravenously (injection or infusion) or intramuscularly |
| Contraindications | Improper for therapy for pernicious anemia and other megaloblastic anemias secondary to the lack of vitamin B12. A hematologic remission may occur while neurologic manifestations continue to progress. |
Known hypersensitivity to calcium folinate, or any of the excipients. Pernicious anemia or other anemias due to vitamin B12 deficiency. |
| Storage Conditions | Store at room temperature, 15 - 30°C | Store at 2 - 8°C |
| Warnings and precautions | See package insert comparison above | See package insert comparison above |
- Wholesalers can place orders directly with Teva using normal procedures.
- Customers can order directly from Teva by contacting Customer Service at (800) 545-8800 between the hours of 8 am and 5 pm EST.
- Calcium Folinate Solution for Injection will be covered under Teva’s Return Goods Policy.
Teva will make reasonable attempts to fill your orders. Teva will be closely monitoring the distribution of Calcium Folinate Solution for Injection to help manage the supply.
To report adverse events among patients administered, please call (215) 293-6351 or (866) 832-8537 between the hours of 8 am and 5 pm EST, or email drug.safety@tevausa.com.
Alternatively, adverse events that may be related to the use of these products may be reported to the FDA’s Med Watch program by fax at 1/800/FDA-0178, by mail at Med Watch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787, or on the Med Watch website at www.fda.gov/Safety/MedWatch.
Sincerely,
Deborah A. Jaskot, M.S., RAC
Vice President, North America Regulatory Affairs
Policy & Governance
| CALCIUM FOLINATE
calcium folinate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CALCIUM FOLINATE
calcium folinate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Teva Parenteral Medicines, Inc (794362533) |
More about leucovorin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antidotes
- Breastfeeding