Brisdelle: Package Insert / Prescribing Info
Package insert / product label
Generic name: paroxetine
Dosage form: capsule
Drug class: Selective serotonin reuptake inhibitors
Medically reviewed by Drugs.com. Last updated on May 9, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
BRISDELLE® (paroxetine) capsules, for oral use
Initial U.S. Approval: 1992
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
Antidepressants, including selective serotonin reuptake inhibitors (SSRIs), increased the risk of suicidal thoughts and behavior in pediatric and young adult patients with major depressive disorder and other psychiatric disorder. Because BRISDELLE is an SSRI, closely monitor patients for clinical worsening and for emergence of suicidal thoughts and behaviors. BRISDELLE is not approved for use in pediatric and young adult patients (5.1).
Indications and Usage for Brisdelle
Brisdelle Dosage and Administration
- The recommended dosage of BRISDELLE is 7.5 mg once daily, at bedtime (2.1)
Dosage Forms and Strengths
Capsules: 7.5 mg (3)
Contraindications
Warnings and Precautions
- Suicidality: Monitor for suicidality or unusual changes in behavior (5.1)
- Serotonin Syndrome: BRISDELLE can cause serotonin syndrome with increased risk when co-administered with other serotonergic agents, but also when taken alone. If it occurs, discontinue BRISDELLE and serotonergic agents and initiate supportive measures (5.2, 7.3).
- Tamoxifen: Efficacy of tamoxifen may be reduced when administered concomitantly with BRISDELLE (5.3, 7.1)
- Abnormal Bleeding: Caution patients about the risk of bleeding associated with the concomitant use of BRISDELLE and non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, or other drugs that affect coagulation (5.4, 7.1)
- Angle-Closure Glaucoma: Angle closure glaucoma has occurred in patients who have untreated anatomically narrow angles and who are treated with antidepressants. (5.5)
- Hyponatremia: Can occur in association with syndrome of inappropriate antidiuretic hormone secretion (SIADH) (5.6)
- Bone Fracture: Epidemiological studies have reported an association between SSRI treatment and fractures (5.7)
- Activation of Mania/Hypomania: Screen for bipolar disorder and monitor for mania/ hypomania (5.8)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold (5.9)
- Sexual Dysfunction: BRISDELLE use may cause symptoms of sexual dysfunction. (5.12)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 2%) reported in clinical trials were: headache, fatigue, and nausea/vomiting (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Legacy Pharma Inc. at 1-800-727-7151 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
Paroxetine is a strong CYP2D6 inhibitor. Co-administration of BRISDELLE can alter concentrations of other drugs that are metabolized by CYP2D6. Consider potential drug interactions prior to and during therapy (5.3, 7.1, 7.3). See Full Prescribing Information for a list of clinically significant drug interactions (7.1, 7.2, 7.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2025
Full Prescribing Information
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Selective serotonin reuptake inhibitors (SSRIs) increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term trials for the treatment of major depressive disorder and other psychiatric disorders. Because BRISDELLE is an SSRI, closely monitor BRISDELLE-treated patients closely for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. BRISDELLE is not approved for use in any psychiatric condition or in pediatric and young adult patients [see Indications and Usage (1) and Use in Specific Populations (8.4)].
1. Indications and Usage for Brisdelle
BRISDELLE is indicated for the treatment of moderate to severe vasomotor symptoms (VMS) associated with menopause.
Limitations of Use:
BRISDELLE is not indicated for the treatment of any psychiatric condition. BRISDELLE has a lower recommended paroxetine dosage than that used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, generalized anxiety disorder, social anxiety disorder, and post-traumatic stress disorder. The safety and effectiveness of the lower BRISDELLE dosage has not been established for any psychiatric condition. Patients who require paroxetine for treatment of a psychiatric condition should discontinue BRISDELLE and initiate a paroxetine-containing product that is indicated for such use.
2. Brisdelle Dosage and Administration
2.1 Recommended Dosage
The recommended oral dosage of BRISDELLE for the treatment of moderate to severe VMS associated with menopause is 7.5 mg once daily, at bedtime, with or without food [see Clinical Pharmacology (12.3)].
2.2 Use of BRISDELLE Before or After a Monoamine Oxidase Inhibitor
Wait at least 14 days after discontinuation of a monoamine oxidase inhibitor (MAOI) before initiating therapy with BRISDELLE. Conversely, allow at least 14 days after stopping BRISDELLE before starting an MAOI [see Contraindications (4.1), Warnings and Precautions (5.2) and Drug Interactions (7.3)].
3. Dosage Forms and Strengths
BRISDELLE is available as 7.5 mg pink capsules printed with black edible ink with “BRISDELLE” and “7.5 mg” on the capsule. Each capsule contains 9.69 mg of paroxetine mesylate equivalent to 7.5 mg paroxetine base.
4. Contraindications
BRISDELLE is contraindicated in patients:
- Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of an increased risk of serotonin syndrome [see Warnings and Precautions (5.2), Drug Interaction (7)].
- Taking thioridazine because of risk of QT prolongation [see Warnings and Precautions (5.3), Drug Interaction (7)].
- Taking pimozide because of risk of QT prolongation [see Warnings and Precautions (5.3), Drug Interaction (7)].
- With known hypersensitivity (e.g., anaphylaxis, angioedema, Stevens-Johnson syndrome) to paroxetine or to any of the inactive ingredients in BRISDELLE [see Adverse Reactions (6.2)]
- Who are or become pregnant because menopausal VMS does not occur during pregnancy and BRISDELLE may cause fetal harm [see Use in Specific Populations (8.1)].
5. Warnings and Precautions
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
SSRIs increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term trials for the treatment of major depressive disorder (MDD) and other psychiatric disorders - BRISDELLE is not approved for use in any psychiatric condition or in pediatric and young adult patients [see Indications and Usage (1) and Use in Specific Populations (8.4)]. There is limited information regarding suicidal thoughts and behaviors in females who use BRISDELLE for treatment of moderate to severe VMS associated with menopause. The BRISDELLE trials excluded females with a presence or history of previous psychiatric disorders.
Monitor all BRISDELLE-treated patients for any emergence of suicidal thoughts and behaviors, especially during the initial few months of BRISDELLE therapy. Counsel family members to monitor for changes in behavior and to alert the health care provider if such changes occur. Consider discontinuing BRISDELLE in patients who experience emergent suicidal thoughts or behaviors or symptoms that might be precursors to suicidal thoughts or behaviors, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
5.2 Serotonin Syndrome
BRISDELLE can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic agents (including triptans, tricyclic antidepressants, fentanyl, tramadol, meperidine, methadone, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., monoamine oxidase inhibitors (MAOIs) [see Contraindications (4), Drug Interactions (7.3)]. Serotonin syndrome can also occur when BRISDELLE is used alone.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of BRISDELLE with MAOIs is contraindicated. Do not start BRISDELLE in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dosage range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral or local tissue injection) or at lower dosages. If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking BRISDELLE, The patient should be taken off BRISDELLE before initiating treatment with the MAOI [see Contraindications (4.1)]. If concomitant use of BRISDELLE with other serotonergic drugs (besides MAOIs) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during BRISDELLE initiation [see Contraindications (4.1) Drug Interactions (7.3)].
Monitor all patients taking BRISDELLE for the emergence of serotonin syndrome. Discontinue BRISDELLE and any concomitant serotonergic agents immediately if the above events occur and initiate supportive symptomatic treatment.
5.3 Potential Impact on Tamoxifen Efficacy
Some studies have shown that the efficacy of tamoxifen, as measured by the risk of breast cancer relapse/mortality, may be reduced when concomitantly administered with paroxetine as a result of paroxetine’s irreversible inhibition of CYP2D6 and lower tamoxifen blood levels [see Drug Interactions (7.1)]. However, other studies have failed to demonstrate such a risk.
When tamoxifen is used for the treatment or prevention of breast cancer, weigh the likely benefit of BRISDELLE for treating moderate to severe VMS associated with menopause vs. the risk of possible decreased tamoxifen effectiveness, and consider avoiding the concomitant use of BRISDELLE.
5.4 Increased Risk of Bleeding
SSRIs, including BRISDELLE, increased the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may add to this risk [see Drug Interactions (7.3)]. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the risk of bleeding associated with the concomitant use of BRISDELLE and antiplatelet agents or anticoagulants [see Drug Interactions (7.1)]. For patients taking warfarin, carefully monitor the international normalized ratio.
5.5 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of SSRIs, including BRISDELLE, may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Cases of angle-closure glaucoma associated with use of paroxetine have been reported. Avoid use of SSRIs, including BRISDELLE, in patients with untreated anatomically narrow angles.
5.6 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs, including BRISDELLE. Cases with serum sodium lower than 110 mmol/L have been reported in patients using SSRIs. Geriatric patients, patients taking diuretics, and those who are volume-depleted may be at greater risk of developing hyponatremia with SSRIs [see Use in Specific Populations (8.5)]. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death. In many cases, the hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
In patients with symptomatic hyponatremia, discontinue BRISDELLE and institute appropriate medical intervention.
5.7 Bone Fracture
Epidemiological studies on bone fracture risk following exposure to SSRIs have reported an association between SSRI treatment and fractures. It is unknown to what extent fracture risk is directly attributable to SSRI treatment. If a BRISDELLE-treated patient presents with unexplained bone pain, point tenderness, swelling, or bruising, consider the possibility of a fragility fracture.
5.8 Screening Patients for Bipolar Disorder and Monitoring for Mania/Hypomania
BRISDELLE is only indicated for the treatment of moderate to severe VMS and is not approved for use in treating either depression or bipolar depression. However, prior to initiating treatment with BRISDELLE, all patients should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It is generally believed (though not established in controlled trials) that use of an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder.
5.9 Seizures
In clinical studies of another paroxetine product, seizures occurred in 0.1% of paroxetine-treated patients.
Use BRISDELLE cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold. Discontinue BRISDELLE in any patient who develops seizures.
5.10 Sexual Dysfunction
Use of SSRIs, including BRISDELLE, may cause symptoms of sexual dysfunction. In female patients, SSRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of BRISDELLE and to inquire specifically about changes in sexual function during treatment because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes. Discuss potential management strategies to support patients in making informed decisions about treatment.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed elsewhere in labeling:
- Suicidality [see Warnings and Precautions (5.1)]
- Serotonin syndrome [see Warnings and Precautions (5.2)]
- Abnormal bleeding [see Warnings and Precautions (5.4)]
- Angle-Closure Glaucoma [see Warnings and Precautions (5.5)]
- Hyponatremia [see Warnings and Precautions (5.6)]
- Bone Fracture [see Warnings and Precautions (5.7)]
- Mania/Hypomania [see Warnings and Precautions (5.8)]
- Seizure [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot directly be compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to BRISDELLE in the following randomized, placebo-controlled trials for the treatment of moderate to severe VMS associated with menopause [see Clinical Studies (14)]: (1) one 8-week Phase 2 trial, (2) one 12-week Phase 3 trial and (3) one 24-week Phase 3 trial. In these trials, a total of 635 postmenopausal females received BRISDELLE 7.5 mg administered orally once daily and 641 postmenopausal females received placebo. In these trials, 68% were White, 30% were Black or African American (30%), and 2% were other races, with a mean age of 55 years (range 40 to 73 years). Postmenopausal females with a history of suicidal ideation or suicidal behavior were excluded from these trials.
Serious Adverse Reactions:
In the pooled Phase 2 and Phase 3 trials, three BRISDELLE-treated patients reported a serious adverse reaction of suicidal ideation and one BRISDELLE-treated patient reported a serious adverse reaction of suicide attempt. There were no serious adverse reactions of suicidal ideation or suicide attempt reported among the placebo-treated patients.
Adverse Reactions Leading to Study Discontinuation:
A total of 4.7% of females taking BRISDELLE discontinued from the clinical trials due to an adverse reaction, compared to 3.7% of females on placebo; the most frequent adverse reactions leading to discontinuation among paroxetine-treated females were: abdominal pain (0.3%), attention disturbances (0.3%), headache (0.3%), and suicidal ideation (0.3%).
Common Adverse Reactions:
Overall, based on investigators’ determinations about what events were likely to be drug-related, about 20% of postmenopausal females treated with BRISDELLE reported at least 1 adverse reaction in the three controlled trials. The most common adverse reactions (≥ 2% and at a higher incidence in BRISDELLE-treated females compared to placebo-treated females) reported in these trials were headache, fatigue/malaise/lethargy, and nausea/vomiting. Of these commonly reported adverse reactions, nausea occurred primarily within the first 4 weeks of BRISDELLE treatment and fatigue occurred primarily within the first week of BRISDELLE treatment, and decreased in frequency with continued therapy.
The adverse reactions that occurred in ≥ 2% of BRISDELLE-treated patients and at a higher incidence in BRISDELLE-treated females compared to placebo-treated females are shown in Table 1 for the pooled Phase 2 and Phase 3 trials.
Table 1: Incidence of Common Adverse Reactions in the Phase 2 and Phase 3 Trials of Postmenopausal Females with Moderate to Severe VMS1
|
Incidence n (%) |
||
|
BRISDELLE (n = 635) |
BRISDELLE (n = 641) |
|
|
Headache |
40 (6.3) |
31 (4.8) |
|
Fatigue, malaise, lethargy |
31 (4.9) |
18 (2.8) |
|
Nausea, vomiting |
27 (4.3) |
15 (2.3) |
1 ≥ 2% BRISDELLE-treated patients and at a higher incidence in BRISDELLE-treated females compared to placebo-treated females.
Adverse Reactions After Discontinuing BRISDELLE
Certain symptoms were seen more frequently in postmenopausal females at the time of discontinuation of BRISDELLE compared to discontinuation of placebo and have also been reported upon discontinuation of other paroxetine products, particularly after abrupt discontinuation. Adverse reactions reported after discontinuation of BRISDELLE included increased dreaming/nightmares, muscle cramps/spasms/twitching, headache, nervousness/anxiety, fatigue/tiredness, restless feeling in legs, and trouble sleeping/insomnia. While these adverse reactions were generally self-limiting, there have been reports of serious discontinuation symptoms with other paroxetine products.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of this and other paroxetine products. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Idiopathic thrombocytopenic purpura, Events related to impaired hematopoiesis (including aplastic anemia, pancytopenia, bone marrow aplasia, agranulocytosis).
Cardiac Disorders: Atrial fibrillation, Pulmonary edema, Ventricular fibrillation, Ventricular tachycardia (including torsades de pointes).
Gastrointestinal Disorders: Pancreatitis, Pancreatitis hemorrhagic, Vomiting.
General Disorders and Administration Site Conditions: Death, Drug withdrawal syndrome, Malaise.
Hepatobiliary Disorders: Drug-induced liver injury, Hepatic failure, Jaundice.
Immune System Disorders: Anaphylaxis, Angioedema, Toxic epidermal necrolysis.
Investigations: Elevated liver tests (the most severe cases were deaths due to liver necrosis, and grossly elevated transaminases associated with severe liver dysfunction).
Metabolism and Nutrition Disorders: Diabetes mellitus inadequate control, Type 2 diabetes mellitus.
Nervous System Disorders: Neuroleptic malignant syndrome, Paresthesia, Somnolence, Tremor, Anosmia, Hyposmia
Psychiatric Disorders: Aggression, Agitation, Anxiety, Confusional state, Depression, Disorientation, Homicidal ideation, Insomnia, Restlessness.
Respiratory, Thoracic and Mediastinal Disorders: Pulmonary hypertension.
Skin and Subcutaneous Tissue Disorders: Hyperhidrosis, Stevens-Johnson syndrome, Drug reaction with eosinophilia and systemic symptoms (DRESS).
Related/similar drugs
7. Drug Interactions
7.1 Potential for BRISDELLE to Affect Other Drugs
Paroxetine is a strong CYP2D6 inhibitor. Clinical drug interaction studies have been performed with substrates of CYP2D6 and show that paroxetine can inhibit the metabolism of drugs metabolized by CYP2D6 [see Clinical Pharmacology (12.3)]. Table 2 contains examples of drugs with a metabolism that may be affected by concomitant use with BRISDELLE.
Table 2 Effects of Paroxetine on Other Drugs
| Concomitant Drug Name | Effect of Paroxetine on Other Drugs | Clinical Recommendations |
| Thioridazine | Increased plasma concentrations of thioridazine Potential QTc prolongation | Concomitant use of thioridazine and BRISDELLE is contraindicated. |
| Pimozide | Increased plasma concentrations of pimozide. Potential QTc prolongation | Concomitant use of pimozide and BRISDELLE is contraindicated. |
| Tamoxifen | Reduced plasma concentrations of active tamoxifen metabolite | Consider avoiding concomitant use of tamoxifen and BRISDELLE. |
| Tricyclic Antidepressants (TCA) (e.g., Desipramine) | Increased plasma concentrations and elimination half-life | Plasma TCA concentrations may need to be monitored and the TCA dosage may need to be reduced if a TCA is used concomitantly with BRISDELLE. Monitor tolerability. |
| Risperidone | Increased plasma concentrations of risperidone | A lower risperidone dosage may be necessary (see the risperidone Prescribing Information for). Monitor tolerability. |
| Atomoxetine | Increased exposure of atomoxetine | A lower atomoxetine dosage of may be necessary (see atomoxetine Prescribing Information for). Monitor tolerability. |
| Drugs Highly Bound to Plasma Protein (e.g., Warfarin) | Increased free plasma concentrations | The warfarin dosage may need to be reduced. Monitor tolerability and the International Normalized Ratio. |
| Digoxin | Decreased plasma concentrations of digoxin | The digoxin dosage of may need to be increased. Monitor digoxin concentrations and clinical effect. |
| Theophylline | Increased plasma concentrations of theophylline | The theophylline dosage of may need to be decreased. Monitor theophylline concentrations and tolerability. |
Use caution with concomitant use of BRISDELLE with other drugs that are metabolized by CYP2D6, including nortriptyline, amitriptyline, imipramine, desipramine, fluoxetine, phenothiazines, risperidone, and Type 1C antiarrhythmics (e.g., propafenone, flecainide, and encainide).
7.2 Potential for Other Drugs to Affect BRISDELLE
The metabolism and pharmacokinetics of paroxetine may be affected by the induction and inhibition of drug metabolizing enzymes such as CYP2D6. Table 3 contains a list of drugs that may affect the pharmacokinetics of BRISDELLE when administered concomitantly [see Clinical Pharmacology (12.3)].
Table 3 Effects of Other Drugs on Paroxetine
| Concomitant Drug Name | Effect of Concomitant Drug on Paroxetine | Clinical Recommendations |
| Phenobarbital | Decreased paroxetine exposure | |
| Phenytoin | Decreased paroxetine exposure | |
| Fosamprenavir/Ritonavir | Decreased plasma concentration of paroxetine | Monitor clinical effect of BRISDELLE. No BRISDELLE dosage adjustment is needed. |
| Cimetidine | Increased plasma concentration of paroxetine |
Use caution if BRISDELLE is used concomitantly with other drugs that inhibit CYP2D6 (e.g., quinidine).
7.3 Other Potentially Significant Drug Interactions
Monoamine Oxidase Inhibitors
Serious adverse reactions such as serotonin syndrome have been reported in patients treated with a SSRI and a concomitant monoamine oxidase inhibitor (MAOI), in patients started on an SSRI who recently received an MAOI and in patients started on an MAOI who recently received an SSRI. Therefore, concomitant use of MAOIs with BRISDELLE or use of BRISDELLE and an MAOI within 14 days of each other is contraindicated [see Dosage and Administration (2.2), Contraindications (4.1) and Warnings and Precautions (5.2)].
Serotonergic Drugs
If concomitant use of BRISDELLE with other serotonergic drugs (e.g., other SNRIs, SSRIs, triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) is clinically warranted, consider the increased risk of serotonin syndrome and carefully observe the patient, particularly during treatment initiation [see Warnings and Precautions (5.2)].
An interaction between paroxetine and tryptophan may occur when they are co-administered. Adverse reactions, consisting primarily of headache, nausea, sweating, and dizziness, have been reported when tryptophan was administered to patients taking paroxetine. Consequently, concomitant use of BRISDELLE with tryptophan is not recommended.
Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs are concomitantly administered with NSAIDs, aspirin, warfarin. or other drugs that affect coagulation. There may be a pharmacodynamic interaction between paroxetine and warfarin that causes an increased bleeding diathesis despite unaltered prothrombin time. Carefully monitor patients receiving warfarin therapy when BRISDELLE is initiated or discontinued [see Warnings and Precautions (5.4)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
BRISDELLE is contraindicated in pregnant females and not indicated for use in pre-menopausal females. Based on epidemiologic and animal studies, paroxetine can cause fetal harm. Based on data from published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Warnings and Precautions (5.4)].
Paroxetine is associated with a less than 2-fold increase in cardiovascular malformations when administered to a pregnant female during the first trimester. While individual epidemiological studies on the association between paroxetine use and cardiovascular malformations have reported inconsistent findings, some meta-analyses of epidemiological studies have identified an increased risk of cardiovascular malformations (see Data). There are risks of persistent pulmonary hypertension of the newborn (PPHN) (see Data) and/or poor neonatal adaptation with exposure to selective serotonin reuptake inhibitors (SSRIs), including BRISDELLE, during pregnancy.
No evidence of treatment related malformations was observed in animal reproduction studies, when paroxetine was administered during the period of organogenesis at doses up to 50 mg/kg/day in rats and 6 mg/kg/day in rabbits. These doses are approximately 64 times (rat) and less than 16 times (rabbit) the maximum recommended human dose (MRHD) of BRISDELLE (7.5 mg) on an mg/m2 basis. When paroxetine was administered to female rats during the last trimester of gestation and continued through lactation, there was an increase in the number of pup deaths during the first four days of lactation. This effect occurred at a dose of 1 mg/kg/day which is 1.3 times the MRHD on an mg/m2 basis (see Data).
Data
Human Data: Published epidemiological studies on the association between first trimester paroxetine use and cardiovascular malformations have reported inconsistent results; however, meta-analyses of population-based cohort studies published between 1996-2017 indicate a less than 2-fold increased risk for overall cardiovascular malformations. Specific cardiac malformations identified in two meta-analyses include approximately 2 to 2.5-fold increased risk for right ventricular outflow tract defects. One meta-analysis also identified an increased risk (less than 2-fold) for bulbus cordis anomalies and anomalies of cardiac septal closure, and an increased risk for atrial septal defects (pooled OR 2.38, 95% CI 1.14-4.97). Important limitations of the studies included in these meta-analyses include potential confounding by indication, depression severity, and potential exposure misclassification.
Exposure to SSRIs, particularly later in pregnancy, may have an increased risk for PPHN. PPHN occurs in 1-2 per 1000 live births in the general population and is associated with substantial neonatal morbidity and mortality.
Animal Data: Reproduction studies were performed at doses up to 50 mg/kg/day in rats and 6 mg/kg/day in rabbits administered during organogenesis. These doses are approximately 64 times (rat) and less than 16 times (rabbit) MRHD of BRISDELLE (7.5 mg) on an mg/m2 basis. These studies have revealed no evidence of malformations. However, in rats, there was an increase in pup deaths during the first 4 days of lactation when dosing occurred during the last trimester of gestation and continued throughout lactation. This effect occurred at a dose of 1 mg/kg/day which is 1.3 times the MRHD on an mg/m2 basis. The no effect dose for rat pup mortality was not determined. The cause of these deaths is not known.
8.4 Pediatric Use
Safety and effectiveness of BRISDELLE in pediatric patients have not been established; BRISDELLE is not indicated in the pediatric population.
8.5 Geriatric Use
Clinical trials of BRISDELLE did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger adult patients. Patients 65 years of age and older may have elevated paroxetine plasma concentrations compared to younger adult patients. However, the recommended BRISDELLE dosage in patients 65 years of age and older is that same as that as younger patients [see Clinical Pharmacology (12.3)].
SSRIs have been associated with cases of clinically significant hyponatremia in geriatric patients, who may be at greater risk for this adverse reaction [see Warnings and Precautions (5.6)].
8.6 Renal Impairment
The recommended BRISDELLE dosage in patients with renal impairment is the same as those with normal renal function [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The recommended BRISDELLE dosage in patients with hepatic impairment is the same as those with normal hepatic function [see Clinical Pharmacology (12.3)].
10. Overdosage
The following have been reported with paroxetine tablets overdose:
- Seizures, which may be delayed, and altered mental status including coma.
- Cardiovascular toxicity, which may be delayed, including QRS and QTc interval prolongation. Hypertension most commonly seen, but rarely can see hypotension alone or with co-ingestants including alcohol.
- Serotonin syndrome (patients with a multiple drug overdosage with other pro-serotonergic drugs may have a higher risk).
Consider contacting the Poison Help Line (1-800-221-2222) or a medical toxicologist for overdose management recommendations.
11. Brisdelle Description
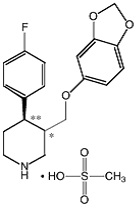
BRISDELLE (paroxetine) is an orally administered selective serotonin reuptake inhibitor (SSRI) for the treatment of moderate to severe VMS associated with menopause. It is identified chemically as (-)-trans-4R- (4’-fluorophenyl) - 3S - [(3’, 4’-methylenedioxyphenoxy) methyl] piperidine mesylate and has the empirical formula of C19H20FNO3•CH3SO3H. The molecular weight is 425.5 (329.4 as free base). The structural formula is:
The mesylate salt of paroxetine is an odorless, off-white powder, having a melting point range of 147° to 150°C and a solubility of more than 1 g/mL in water.
Each pink capsule contains 9.69 mg of paroxetine mesylate equivalent to 7.5 mg paroxetine base.
Inactive ingredients consist of: dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, gelatin, titanium dioxide, FD&C Yellow #6, FD&C Red #3, FD&C Red #40, shellac, and black iron oxide.
12. Brisdelle - Clinical Pharmacology
12.1 Mechanism of Action
Nonclinical studies have shown that paroxetine is an SSRI; BRISDELLE is not an estrogen. The mechanism of action of BRISDELLE for the treatment of moderate to severe VMS associated with menopause is unknown.
Studies at clinically relevant BRISDELLE doses in humans have demonstrated that paroxetine blocks the uptake of serotonin into human platelets. Paroxetine is a neuronal serotonin reuptake inhibitor with weak effects on norepinephrine and dopamine neuronal reuptake in vitro. Paroxetine also has low affinity for muscarinic alpha1-, alpha2-, beta-adrenergic, dopamine (D2)-, 5-HT1-, 5-HT2-, and histamine (H1)-receptors.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of paroxetine for the treatment of moderate to severe VMS associated with menopause have not been fully characterized.
12.3 Pharmacokinetics
Absorption
Paroxetine is completely absorbed after oral dosing.
In a study in which healthy postmenopausal females (n=24) received BRISDELLE 7.5 mg once daily for 14 days, steady-state paroxetine concentrations were achieved by approximately 12 days of dosing for most subjects, although it may take substantially longer in an occasional patient. Peak concentrations were reached at a median of 6 hours (3 to 8 hours range). Steady-state mean values of Cmax, Cmin, and AUC0-last were 13.10 ng/mL (CV 91%), 7.17 ng/mL (CV 99%), and 237 hr*ng/mL (CV 94%), respectively.
Steady-state AUC0-24 values were about 3 times those of AUC0-inf following a single BRISDELLE dose, indicating non-linear pharmacokinetics. Steady-state Cmax values were approximately 5 times greater than those attained after a single BRISDELLE dose and steady-state exposure based on AUC0-24 was about 10 times greater than AUC0-24 after a single dose.
The nonlinear kinetics and excess accumulation are due to the fact that CYP2D6, an enzyme that is in part responsible for paroxetine metabolism, is readily saturable.
Effect of Food: The effects of food on the bioavailability of paroxetine were studied after administration of another paroxetine product with a dosage higher than BRISDELLE. AUC was slightly increased (6%) when paroxetine was administered with food and the Cmax was 29% greater, while the time to reach peak plasma concentration decreased from 6.4 hours post-dosing to 4.9 hours. These changes are not clinically significant [see Dosage and Administration (2.1)].
Distribution
Paroxetine distributes throughout the body, including the central nervous system, with only 1% remaining in the plasma.
Approximately 95% and 93% of paroxetine is bound to plasma protein at 100 ng/mL and 400 ng/mL, respectively. Paroxetine does not alter the in vitro protein binding of phenytoin or warfarin.
Elimination
Metabolism: Paroxetine is extensively metabolized after oral administration. The principal metabolites are polar and conjugated products of oxidation and methylation, which are readily cleared. Conjugates with glucuronic acid and sulfate predominate, and major metabolites have been isolated and identified. Data indicate that the metabolites have no more than 1/50 the potency of the parent compound at inhibiting serotonin uptake. The metabolism of paroxetine is accomplished in part by cytochrome CYP2D6. The role of this enzyme in paroxetine metabolism also suggests potential drug interactions [see Drug Interactions (7)]. At steady state, when the CYP2D6 pathway is essentially saturated, paroxetine clearance is governed by alternative P450 isozymes, which, unlike CYP2D6, show no evidence of saturation.
Excretion: Approximately 64% of a 30 mg oral solution of another paroxetine product (four times the recommended BRISDELLE dosage) was excreted in the urine with 2% as the parent compound and 62% as metabolites over a 10-day post-dosing period. About 36% of the dose was excreted in the feces (probably via the bile), mostly as metabolites and less than 1% as the parent compound over the 10-day post-dosing period.
Specific Populations
Patients with Renal and Hepatic Impairment: Increased plasma concentrations of paroxetine occur in subjects with renal and hepatic impairment. The mean plasma concentration in patients with creatinine clearance below 30 mL/min was approximately 4 times greater than seen in normal volunteers. Patients with creatinine clearance of 30 to 60 mL/min and patients with hepatic impairment had about a 2-fold increase in plasma concentrations (AUC, Cmax) [see Use in Specific Populations (8.6, 8.7)].
Geriatric Patients: In a multiple-dose study in geriatric patients with another paroxetine product at doses of 20, 30, and 40 mg (1.7, 4, and 5.3, times the maximum recommended BRISDELLE dosage, respectively), Cmin concentrations were about 70% to 80% greater than the respective Cmin concentrations in younger adult subjects [see Use in Specific Populations (8.5)].
Drug Interaction Studies
Potential Effect of BRISDELLE on Other Drugs
- Drugs Metabolized by CYP3A4: An in vivo drug interaction study involving the concomitant use of paroxetine and terfenadine, a substrate for cytochrome CYP3A4, under steady-state conditions revealed no effect of paroxetine on terfenadine pharmacokinetics. In vitro studies have shown ketoconazole, a potent CYP3A4 inhibitor, to be at least 100 times more potent than paroxetine as an inhibitor of the metabolism of several substrates for CYP3A4, including astemizole, triazolam, and cyclosporine. Based on the assumption that the relationship between paroxetine’s in vitro Ki and its lack of effect on terfenadine’s in vivo clearance predicts its effect on other CYP3A4 substrates, paroxetine’s extent of CYP3A4 inhibition is not likely to be of clinical significance.
-
Drugs Metabolized by CYP2D6: Many drugs are metabolized by the cytochrome P450 isozyme CYP2D6. Like other agents that are metabolized by CYP2D6, paroxetine may significantly inhibit the activity of this isozyme. In most patients (> 90%), this CYP2D6 isozyme is saturated early during paroxetine dosing. Specific studies investigating the effect of paroxetine on drugs metabolized by CYP2D6 are listed below:
- Pimozide: In a controlled study of healthy volunteers, after another paroxetine product was titrated to 60 mg daily (8 times the maximum recommended BRISDELLE dosage), concomitant use of paroxetine with a single 2 mg dose of pimozide was associated with mean increases in pimozide AUC of 151% and Cmax of 62%, compared to pimozide administered alone [see Drug Interactions (7.1)].
- Desipramine: In one study, daily dosing of another paroxetine product (20 mg once daily) (2.7 times the recommended BRISDELLE dosage) under steady-state conditions with a concomitant single dose of desipramine (100 mg) increased desipramine Cmax, AUC, and T½ by an average of approximately 2-, 5-, and 3-fold, respectively [see Drug Interactions (7.1)].
- Risperidone: Daily dosing of another paroxetine product at 20 mg in patients stabilized on risperidone (4 to 8 mg/day), a CYP2D6 substrate, increased mean plasma risperidone concentrations approximately 4-fold, decreased 9-hydroxyrisperidone concentrations approximately 10%, and increased concentrations of the active moiety (the sum of risperidone plus 9-hydroxyrisperidone) approximately 1.4-fold [see Drug Interactions (7.1)].
- Atomoxetine: The effect of paroxetine on the pharmacokinetics of atomoxetine has been evaluated when both drugs were at steady state. In healthy volunteers who were extensive metabolizers of CYP2D6, 20 mg daily of another paroxetine product was given in combination with 20 mg atomoxetine every 12 hours resulting in increases in steady-state atomoxetine AUC values that were 6- to 8-fold greater and in atomoxetine Cmax values that were 3- to 4-fold greater than when atomoxetine was given alone [see Drug Interactions (7.1)].
- Digoxin: Mean digoxin AUC at steady state decreased by 15% in the presence of paroxetine [see Drug Interactions (7.1)].
- Beta Blockers: In a study in which propranolol (80 mg twice daily) was dosed orally for 18 days, the steady-state plasma concentrations of propranolol were unaltered during concomitant use with another paroxetine product (30 mg once daily) (4 times the recommended BRISDELLE dosage) for the final 10 days. The effects of propranolol on paroxetine have not been evaluated.
Potential Effect of Other Drugs on BRISDELLE
Concomitant use of paroxetine with other drugs that alter CYP enzymes activities including CYP2D6 may affect the plasma concentrations of paroxetine. Specific studies investigating the effect of other drugs on paroxetine are listed below:
- Cimetidine: Cimetidine inhibits many cytochrome P450 enzymes. In a study in which another paroxetine product was dosed orally at 30 mg once daily (4 times the recommended BRISDELLE dosage) for 4 weeks, steady-state plasma concentrations of paroxetine were increased by approximately 50% during concomitant use with oral cimetidine (300 mg three times daily) for the final week [see Drug Interactions (7.2)].
- Phenobarbital: Phenobarbital induces many cytochrome P450 enzymes. When a single oral 30 mg dose of another paroxetine product (4 times the recommended BRISDELLE dosage) was administered at phenobarbital steady state (100 mg once daily for 14 days), paroxetine AUC and T½ were reduced (by an average of 25% and 38%, respectively) compared to paroxetine administered alone. The effect of paroxetine on phenobarbital pharmacokinetics was not studied. Because paroxetine exhibits nonlinear pharmacokinetics, the results of this study may not address the case where the two drugs are both being chronically dosed [see Drug Interactions (7.2)].
- Phenytoin: When a single oral 30 mg dose of another paroxetine product (4 times the recommended BRISDELLE dosage) was administered at phenytoin steady state (300 mg once daily for 14 days), paroxetine AUC and T½ were reduced (by an average of 50% and 35%, respectively) compared to paroxetine administered alone. In a separate study, when a single oral 300 mg dose of phenytoin was administered with another paroxetine product (30 mg once daily for 14 days) at paroxetine steady state, phenytoin AUC was slightly reduced (12% on average) compared to phenytoin administered alone. Because both drugs exhibit nonlinear pharmacokinetics, the above studies may not address the case where the two drugs are both being chronically dosed [see Drug Interactions (7.2)].
- Digoxin: A clinical drug interaction study showed that concurrent use of digoxin did not affect paroxetine exposure.
- Diazepam: A clinical drug interaction study showed that concurrent use of diazepam did not affect paroxetine exposure.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two-year carcinogenicity studies were conducted in rodents given paroxetine in the diet at 1, 5, and 25 mg/kg/day (mice) and 1, 5, and 20 mg/kg/day (rats). The doses used in these carcinogenicity studies were approximately 16 (mouse) and 26 (rat) times the MHRD for BRISDELLE for the treatment of moderate to severe VMS associated with menopause. There was a significantly greater number of male rats in the high-dose group with reticulum cell sarcomas (1/100, 0/50, 0/50, and 4/50 for control, low-, middle-, and high-dose groups, respectively) and a significantly increased linear trend across groups for the occurrence of lymphoreticular tumors in male rats. Female rats were not affected. Although there was a dose-related increase in the number of tumors in mice, there was no paroxetine -related increase in the number of mice with tumors. The relevance of these findings to humans is unknown.
Mutagenesis
Paroxetine produced no genotoxic effect in a battery of 5 in vitro and 2 in vivo assays that included the following: bacterial mutation assay, mouse lymphoma mutation assay, unscheduled DNA synthesis assay, and tests for cytogenetic aberrations in vivo in mouse bone marrow and in vitro in human lymphocytes and in a dominant lethal test in rats.
14. Clinical Studies
The effectiveness of BRISDELLE as a treatment for moderate to severe vasomotor symptoms (VMS) associated with menopause was established in two Phase 3 randomized, double-blind, placebo-controlled clinical trials in 1,174 postmenopausal females. In these trials, patients must have had a minimum of 7-8 moderate to severe VMS per day at baseline (≥ 50 per week) for 30 days prior to receiving study drug. Patients were randomized to BRISDELLE 7.5 mg orally once daily or daily placebo:
- Trial 1 was a 12-week trial with a total of 606 postmenopausal females (average age 55 years; 65% White, 33% Black or African American, and 2% other races; 10.6% were Hispanic/Latina and 89.4% were not Hispanic/Latina; 18% surgically menopausal and 82% naturally menopausal).
- Trial 2 was a 24-week trial with a total of 568 postmenopausal females (average age 54 years; 76% White, 22% Black or African American, and 2% other races; 6.5% were Hispanic/Latina and 93.5% were not Hispanic/Latina; 20% surgically menopausal and 81% naturally menopausal).
The co-primary efficacy endpoints for both trials were the reduction from baseline in VMS frequency and VMS severity at Weeks 4 and 12.
- Data from Trial 1 showed a statistically significant reduction from baseline in the frequency of moderate to severe VMS at Week 4 and Week 12 and a statistically significant reduction in the severity of moderate to severe VMS at Week 4 in the BRISDELLE group compared to the placebo group (Table 4).
- Data from Trial 2 showed a statistically significant reduction from baseline in the frequency and severity of moderate to severe VMS at Week 4 and Week 12 in the BRISDELLE group compared to placebo group (Table 5).
Table 4: Trial 1: Changes in the Daily Frequency and Daily Severity of VMS at Weeks 4 and 12 in Postmenopausal Females with Moderate to Severe VMS (MITT Population)
| Frequency | Severity | |||
| BRISDELLE | Placebo | BRISDELLE | Placebo | |
| Baseline | ||||
| n | 301 | 305 | 301 | 305 |
| Median | 10.4 | 10.4 | 2.5 | 2.5 |
| Change from baseline at Week 4 | ||||
| n | 289 | 293 | 281 | 289 |
| Median | -4.3 | -3.1 | -0.05 | 0.00 |
| Treatment Difference* | -1.2 | -0.05 | ||
| P-value# | <0.01 | <0.01 | ||
| Change from baseline at Week 12 | ||||
| n | 264 | 274 | 236 | 253 |
| Median | -5.9 | -5.0 | -0.06 | -0.02 |
| Treatment Difference* | -0.9 | -0.04 | ||
| P-value# | <0.01 | 0.17 | ||
MITT population: all consented and randomized patients with valid baseline daily hot flash diary data who had taken at least 1 dose of study drug and had at least 1 day of on-treatment daily hot flash diary data.
* Treatment Difference: the difference between the median changes from baseline.
# P-value was obtained from rank-ANCOVA model.
Table 5: Trial 2: Changes in the Daily Frequency and Daily Severity of VMS at Weeks 4 and 12 in Postmenopausal Females with Moderate to Severe VMS (MITT Population)
| Frequency | Severity | |||
| BRISDELLE | Placebo | BRISDELLE | Placebo | |
| Baseline | ||||
| n | 284 | 284 | 284 | 284 |
| Median | 9.9 | 9.6 | 2.5 | 2.5 |
| Change from baseline at Week 4 | ||||
| n | 276 | 274 | 268 | 271 |
| Median | -3.8 | -2.5 | -0.04 | -0.01 |
| Treatment Difference* | -1.3 | -0.03 | ||
| P-value# | <0.01 | 0.04 | ||
| Change from baseline at Week 12 | ||||
| n | 257 | 244 | 245 | 236 |
| Median | -5.6 | -3.9 | -0.05 | 0.00 |
| Treatment Difference* | -1.7 | -0.05 | ||
| P-value# | <0.01 | <0.01 | ||
MITT population: all consented and randomized patients with valid baseline daily hot flash diary data who had taken at least 1 dose of study drug and had at least 1 day of on-treatment daily hot flash diary data.
* Treatment Difference: the difference between the median changes from baseline.
# P-value is obtained from rank-ANCOVA model.
Persistence of benefit at 24 weeks in Trial 2 was evaluated with a responder analysis where responders were defined as those patients who achieved ≥ 50% reduction from baseline in the frequency of moderate to severe VMS at Week 24. The proportion of patients who achieved a ≥ 50% reduction in the frequency of moderate to severe VMS from baseline to Week 24 was 48% in the BRISDELLE group and 36% in the placebo group at Week 24.
16. How is Brisdelle supplied
BRISDELLE (paroxetine) capsules is available as 7.5 mg pink capsules printed with black edible ink with “BRISDELLE” and “7.5 mg”.
NDC 83107-027-30 blister packs of 30
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). Protect from light and humidity.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Instruct patients to read the Medication Guide before starting therapy with BRISDELLE and to reread it each time the prescription is renewed.
Suicidal Thoughts and Behaviors
Advise patients, their families, and their caregivers to look for the emergence of suicidal thoughts and behaviors, especially early during treatment and to alert the health care provider if such changes occur [see Boxed Warning and Warnings and Precautions (5.1)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of BRISDELLE with triptans, tricyclic antidepressants, opioids, linezolid, tramadol, amphetamines, St. John’s Wort, lithium, tryptophan supplements, other serotonergic agents, or antipsychotic drugs. Instruct patients to contact their health care provider, or report to the emergency room, should they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7.3)].
Potential Impact on Tamoxifen Efficacy
Caution patients that efficacy of tamoxifen may be reduced when administered concomitantly and counsel them about the likely benefit of paroxetine for treating VMS vs. the risk of possible decreased tamoxifen effectiveness [see Warnings and Precautions (5.3)].
Increased Risk of Bleeding
Caution patients about the concomitant use of BRISDELLE and NSAIDs, aspirin, warfarin, and other anticoagulants because combined use of drugs that interfere with serotonin reuptake has been associated with an increased risk of bleeding [see Warnings and Precautions (5.4)].
Angle-Closure Claucoma
Advise patients that taking BRISDELLE can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma -existing [See Warnings and Precautions (5.5)].
Hyponatremia
Caution patients about the risk of hyponatremia, particularly elderly patients and those who are taking diuretics or are volume-depleted [see Warnings and Precautions (5.6)].
Bone Fracture
Inform patients that there is the possibility for an increased risk of fracture [see Warnings and Precautions (5.7)].
Screening Patients for Bipolar Disorder and Monitoring for Mania/Hypomania
Advise patients, their families, and their caregivers to observe for signs of activation of mania/hypomania [see Warnings and Precautions (5.8)].
Pregnancy
Advise patients to notify their physician if they become pregnant during therapy [see Contraindications (4.5) and Use in Specific Populations (8.1)].
Drug Interactions
Advise patients to inform their healthcare provider if they are taking, or plan to take, any prescription or over-the-counter drugs, including herbal supplements, because there is a potential for interaction with paroxetine [see Drug Interactions (7)].
Sexual Dysfunction
Advise patients that use of BRISDELLE may cause symptoms of sexual dysfunction in female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (5.10)].
Allergic Reactions
Advise patients to seek medical attention if they develop an allergic reaction such as rash, hives, swelling, or difficulty breathing [see Adverse Reactions (6.2)].
BRISDELLE is a registered trademark of Legacy Pharma Inc.
©2025 Legacy Pharma Inc. All rights reserved.
Manufactured for:
Legacy Pharma Inc.
George Town, Grand Cayman
KY1-9012

|
MEDICATION GUIDE BRISDELLE® (bris-del) (paroxetine) capsules |
|
Read this Medication Guide that comes with BRISDELLE before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk with your healthcare provider if there is something you do not understand or want to learn more about. What is the most important information I should know about BRISDELLE? BRISDELLE may cause serious side effects, including:
Call your healthcare provider right away or go to the nearest emergency room if you have any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What is BRISDELLE?
|
|
Who should not take BRISDELLE? Do not take BRISDELLE if you:
|
|
What should I tell my healthcare provider before taking BRISDELLE? Before taking BRISDELLE, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BRISDELLE and some medicines may interact with each other, may not work as well, or may cause serious side effects when taken together. If you take BRISDELLE, you should not take any other medicines that contain paroxetine, including Paxil, Paxil CR and Pexeva. Especially tell your healthcare provider if you take:
Ask your healthcare provider if you are not sure if you are taking any of these medications. Your healthcare provider or pharmacist can tell you if it is safe to take BRISDELLE with your other medicines. Do not start or stop any medicine while taking BRISDELLE without talking to your healthcare provider first. |
|
How should I take BRISDELLE?
|
|
What are the possible side effects of BRISDELLE? BRISDELLE may cause serious side effects, including:
Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
The most common side effects of BRISDELLE include:
Tell your healthcare provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of BRISDELLE. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 |
|
How should I store BRISDELLE?
Keep BRISDELLE and all medicines out of the reach of children |
|
General information about the safe and effective use of BRISDELLE. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BRISDELLE for a condition for which it was not prescribed. Do not give BRISDELLE to other people, even if they have the same condition. It may harm them. You may ask your healthcare provider or pharmacist for information about BRISDELLE that is written for healthcare professionals. |
|
What are the ingredients in BRISDELLE? Active ingredient: paroxetine Inactive ingredients: dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, gelatin, titanium dioxide, FD&C Yellow #6, FD&C Red #3, FD&C Red #40, shellac and black iron oxide. Manufactured for: Legacy Pharma Inc., George Town, Grand Cayman BRISDELLE is a registered trademark of Legacy Pharma Inc. ©2025 Legacy Pharma Inc. All rights reserved. For more information about BRISDELLE go to www.BRISDELLE.com MDG90757001 |
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 02/2025
| BRISDELLE
paroxetine capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Legacy Pharma USA, Inc. (118831776) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Packaging Coordinators, LLC | 078525133 | pack(83107-027) , label(83107-027) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Norwich Pharmaceuticals, Inc. | 132218731 | manufacture(83107-027) , analysis(83107-027) , pack(83107-027) , label(83107-027) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Excella GmbH | 329809800 | api manufacture(83107-027) , analysis(83107-027) , manufacture(83107-027) | |
Frequently asked questions
- SSRIs vs SNRIs - What's the difference between them?
- Can paroxetine be used for premature ejaculation?
- What are some common side effects of antidepressants?
More about Brisdelle (paroxetine)
- Check interactions
- Compare alternatives
- Reviews (38)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- FDA approval history
- Drug class: selective serotonin reuptake inhibitors
- Breastfeeding