Beyfortus: Package Insert / Prescribing Info
Package insert / product label
Generic name: nirsevimab
Dosage form: injection
Drug class: Antiviral monoclonal antibodies
Medically reviewed by Drugs.com. Last updated on Sep 2, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
BEYFORTUS (nirsevimab-alip) injection, for intramuscular use
Initial U.S. Approval: 2023
Recent Major Changes
Warnings and Precautions (5.1) 02/2024
Indications and Usage for Beyfortus
BEYFORTUS is a respiratory syncytial virus (RSV) F protein‑directed fusion inhibitor indicated for the prevention of RSV lower respiratory tract disease in:
Beyfortus Dosage and Administration
Administer as an intramuscular injection. (2.1)
Recommended dosage:
Neonates and infants born during or entering their first RSV season:
- •
- 50 mg if less than 5 kg in body weight. (2.1)
- •
- 100 mg if greater than or equal to 5 kg in body weight. (2.1)
Children who remain vulnerable through their second RSV season:
- •
- 200 mg (2 x 100 mg injections). (2.1)
Dosage Forms and Strengths
Contraindications
BEYFORTUS is contraindicated in infants and children with a history of serious hypersensitivity reactions, including anaphylaxis, to nirsevimab-alip or to any of the excipients. (4)
Warnings and Precautions
- •
- Hypersensitivity Reactions Including Anaphylaxis: Serious hypersensitivity reactions have been reported following BEYFORTUS administration. These reactions included urticaria, dyspnea, cyanosis, and/or hypotonia. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reactions occur, initiate appropriate treatment. (5.1)
Adverse Reactions/Side Effects
Most common adverse reactions were rash (0.9%) and injection site reactions (0.3%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi at 1-855-239-3678 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
The safety and effectiveness of BEYFORTUS in children older than 24 months of age have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2024
Full Prescribing Information
1. Indications and Usage for Beyfortus
BEYFORTUS is indicated for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in:
- •
- Neonates and infants born during or entering their first RSV season.
- •
- Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
2. Beyfortus Dosage and Administration
2.1 Recommended Dosage
First RSV Season for Neonates and Infants
For neonates and infants born during the RSV season, administer BEYFORTUS starting from birth. For neonates and infants born outside the RSV season, administer BEYFORTUS once prior to the start of the RSV season considering duration of protection provided by BEYFORTUS [see Clinical Pharmacology (12.2)].
The recommended dosage of BEYFORTUS for neonates and infants born during or entering their first RSV season is based on body weight (see Table 1) and is administered as a single intramuscular (IM) injection.
|
Body Weight at Time of Dosing |
Recommended Dosage |
|
Less than 5 kg |
50 mg by IM injection |
|
5 kg and greater |
100 mg by IM injection |
Second RSV Season for Children Who Remain at Increased Risk for Severe RSV Disease
For children up to 24 months of age, regardless of body weight, who remain at increased risk for severe RSV disease in their second RSV season, refer to Table 2 below for recommended dosage.
|
|
|
Child’s Age at Time of Dosing |
Recommended Dosage |
|
Up to 24 months of age* |
200 mg administered as two IM injections of (2 x 100 mg) |
First and Second RSV Season for Children Undergoing Cardiac Surgery with Cardiopulmonary Bypass
For children undergoing cardiac surgery with cardiopulmonary bypass, an additional dose of BEYFORTUS is recommended as soon as the child is stable after surgery to ensure adequate nirsevimab-alip serum levels. The recommended dosage of BEYFORTUS is administered as an IM injection.
First RSV season:
- •
- If surgery is within 90 days after receiving BEYFORTUS, the additional dose should be based on body weight at the time of the additional dose. Refer to Table 1 for weight-based dosing.
- •
- If more than 90 days have elapsed since receiving BEYFORTUS, the additional dose should be 50 mg regardless of body weight.
Second RSV season:
- •
- If surgery is within 90 days after receiving BEYFORTUS, the additional dose should be 200 mg, regardless of body weight.
- •
- If more than 90 days have elapsed since receiving BEYFORTUS, the additional dose should be 100 mg, regardless of body weight.
2.2 Administration Instructions
BEYFORTUS must be administered by a healthcare provider.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. BEYFORTUS is a clear to opalescent, colorless to yellow solution. Do not inject BEYFORTUS if the liquid is cloudy, discolored, or it contains large particles or foreign particulate matter.
Do not use if the BEYFORTUS pre-filled syringe has been dropped or damaged, the security seal on the carton has been broken, or the expiration date has passed.
BEYFORTUS is available in a 50 mg and a 100 mg pre-filled syringe. Check the labels on the BEYFORTUS carton and pre-filled syringe to ensure the correct 50 mg or 100 mg product is being used.
Co-administration with Childhood Vaccines and Immunoglobulin Products
BEYFORTUS can be given concomitantly with childhood vaccines [see Clinical Pharmacology (12.3)]. When administered concomitantly with injectable vaccines, they should be given with separate syringes and at different injection sites. Do not mix BEYFORTUS with any vaccines or medications in the same syringe or vial.
There is no information regarding co-administration of BEYFORTUS with other immunoglobulin products. Palivizumab should not be administered to infants who have already received BEYFORTUS in the same season. There are no data regarding substitution of BEYFORTUS for palivizumab once prophylaxis treatment is initiated with palivizumab for the RSV season. BEYFORTUS may be administered prior to or during the second RSV season to children up to 24 months of age who remain vulnerable to severe RSV disease, and who received palivizumab in their first RSV season [see Adverse Reactions (6.1) and Clinical Studies (14.3)].
Administration Instructions for Single-Dose Pre-filled Syringe
|
BEYFORTUS 50 mg (50 mg/0.5 mL) pre‑filled syringe with a purple plunger rod. |
BEYFORTUS 100 mg (100 mg/mL) pre‑filled syringe with a light blue plunger rod. |
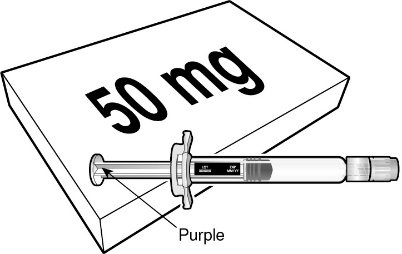 | 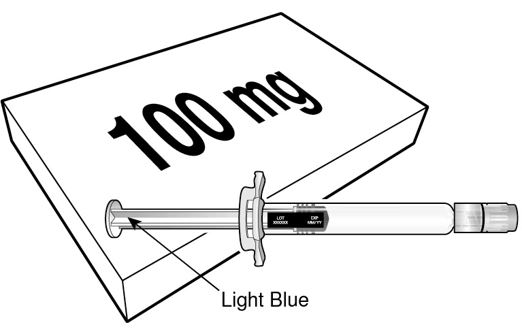 |
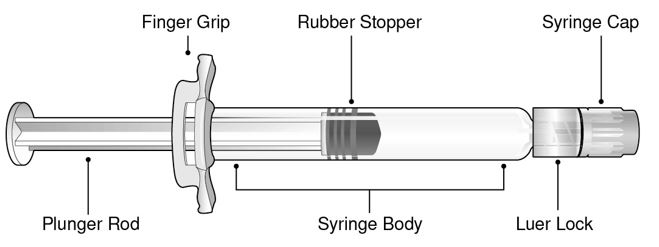
Refer to Figure 1 for pre-filled syringe components.
- Figure 1 Luer Lock Syringe Components
Step 1: Holding the Luer lock in one hand (avoid holding the plunger rod or syringe body), unscrew the syringe cap by twisting it counter‑clockwise with the other hand.
Step 2: Attach a Luer lock needle to the pre-filled syringe by gently twisting the needle clockwise onto the pre-filled syringe until slight resistance is felt.
Step 3: Hold the syringe body with one hand and carefully pull the needle cover straight off with the other hand. Do not hold the plunger rod while removing the needle cover or the rubber stopper may move. Do not touch the needle or let it touch any surface. Do not recap the needle or detach it from the syringe.
Step 4: Administer the entire contents of the BEYFORTUS pre-filled syringe as an IM injection, preferably in the anterolateral aspect of the thigh. The gluteal muscle should not be used as an injection site because of the risk of damage to the sciatic nerve.
Step 5: Discard syringe into a sharps container.
If two injections are required, repeat Steps 1-5 in a different injection site.
3. Dosage Forms and Strengths
BEYFORTUS is a clear to opalescent, colorless to yellow solution available as follows:
- •
- Injection: 50 mg/0.5 mL in a single-dose pre-filled syringe.
- •
- Injection: 100 mg/mL in a single-dose pre-filled syringe.
4. Contraindications
BEYFORTUS is contraindicated in infants and children with a history of serious hypersensitivity reactions, including anaphylaxis, to nirsevimab-alip or to any of the excipients [see Warnings and Precautions (5.1) and Description (11)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions Including Anaphylaxis
Serious hypersensitivity reactions have been reported following BEYFORTUS administration. These reactions included urticaria, dyspnea, cyanosis, and/or hypotonia. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reactions occur, initiate appropriate treatment.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 3,224 pediatric subjects received the recommended dose of BEYFORTUS in Phase 2 and Phase 3 clinical trials (Trials 03, 04, and 05) including 2,119 infants who were born at 35 weeks gestational age (GA) or older, and 1,105 infants who were born at less than 35 weeks GA. A total of 247 infants of any GA with chronic lung disease (CLD) of prematurity or hemodynamically significant congenital heart disease (CHD) in Trial 05 received the recommended dose of BEYFORTUS.
Neonates and Infants Entering Their First RSV Season (Trial 03 and Trial 04)
Trial 03 was a randomized, double-blind placebo-controlled trial conducted in preterm infants born at a GA of greater than or equal to 29 weeks to less than 35 weeks. Subjects were randomized 2:1 to receive BEYFORTUS (N=968) or placebo (N=479) by IM injection. All subjects randomized to BEYFORTUS received a single 50 mg IM dose regardless of body weight. Safety data in Trial 03 are presented only for the infants in the BEYFORTUS arm who received the recommended dose [infants who weighed less than 5 kg and who received a single dose of 50 mg BEYFORTUS IM (N=572) or placebo (N=288)].
Trial 04 was a Phase 3, randomized, double-blind, placebo-controlled trial conducted in late preterm and term infants born at greater than or equal to 35 weeks GA. Trial 04 enrolled subjects sequentially into two cohorts: the Primary Cohort was used for the primary efficacy analysis [see Clinical Studies (14.3)] and for assessment of safety, and the Safety Cohort was used primarily for safety assessment. All subjects from both cohorts of Trial 04 were included in the safety analysis (BEYFORTUS N=1,997 and placebo N=997). Subjects in Trial 04 weighing less than 5 kg received a single 50 mg IM dose of BEYFORTUS and infants weighing greater than or equal to 5 kg received a single 100 mg IM dose.
Infants who received the recommended dose in Trial 03 and infants in Trial 04 were pooled to evaluate the safety of BEYFORTUS (N=2,570) compared to placebo (N=1,284). At randomization, in this pooled Safety Population from Trials 03 and 04 cohorts, 22% of infants were born at less than 35 weeks GA, 10% of infants were GA greater than or equal to 35 weeks and less than 37 weeks; 68% were GA greater than or equal to 37 weeks; 52% were male; 57% were White; 15% were Black; 4% were American Indian/Alaskan native; 4% were Asian; 1% were Pacific Islander; and 19% were Other or Mixed Race; 30% were Hispanic or Latino; 73% were from Northern Hemisphere; and 53% weighed less than 5 kg. The median age was 2 months; 65% were less than or equal to 3 months; 28% were greater than 3 to less than or equal to 6 months, and 7% were greater than 6 months of age. (Refer to Sections 14.2 and 14.3, Clinical Studies, for a description of the efficacy populations in Trials 03 and 04). In both trials, infants received a single dose of IM BEYFORTUS or placebo on Study Day 1 and were monitored for at least 60 minutes post-dose. Subjects were followed for 360 days post-dose to assess safety. Adverse reactions were reported in 1.2% of subjects who received BEYFORTUS; most (97%) of adverse reactions were mild to moderate in intensity.
Table 3 summarizes the adverse reactions that occurred in Trial 03 and Trial 04 (Safety Population) in subjects who received the recommended dose of BEYFORTUS.
|
||
|
Adverse Reaction |
BEYFORTUS |
Placebo |
|
Rash† (occurring within 14 days post-dose) |
0.9 |
0.6 |
|
Injection site reaction‡ (occurring within 7 days post-dose) |
0.3 |
0 |
Infants Born at <35 Weeks Gestational Age and Infants and Children with CLD of Prematurity or Hemodynamically Significant CHD (Trial 05)
RSV Season One
The safety of BEYFORTUS was evaluated in Trial 05, a randomized, double-blind, palivizumab-controlled multicenter trial in infants at high risk for severe RSV disease. These subjects were randomized 2:1 to receive BEYFORTUS (N=614) or palivizumab (N=304) by IM injection. The 614 infants who received BEYFORTUS included 128 preterm infants born at GA less than 29 weeks, 390 preterm infants who were born at 29 weeks or older to less than 35 weeks GA, and 96 late preterm and term infants born at 35 weeks GA or older. Among infants enrolled during their first RSV season, the number of infants with CLD of prematurity or hemodynamically significant CHD were overall 214 and 103, respectively, regardless of gestational age. Of these, 12 infants had both CLD and CHD.
Subjects in Trial 05 weighing less than 5 kg received a single 50 mg IM dose of BEYFORTUS and infants weighing greater than or equal to 5 kg received a single 100 mg IM dose. BEYFORTUS was administered once on Study Day 1 followed by 4 monthly IM doses of placebo; palivizumab was administered IM monthly for 5 months. All subjects were monitored for at least 60 minutes post-dose. Subjects were followed for 360 days post-dose to assess safety.
Adverse reactions reported among Trial 05 subjects who received BEYFORTUS in their first RSV season were similar to those reported in subjects who received BEYFORTUS in Trials 03 and 04.
RSV Season Two (Subjects with CLD of Prematurity and Hemodynamically Significant CHD)
Subjects with CLD of prematurity or hemodynamically significant CHD could continue in Trial 05 and receive BEYFORTUS or palivizumab prior to their second RSV season. All subjects who received BEYFORTUS in the first RSV season also received BEYFORTUS in the second RSV season (N=180). Subjects who received palivizumab in the first RSV season were re-randomized to receive BEYFORTUS (N=40) or palivizumab (N=42) in the second RSV season. Safety data were available for 150 days after dosing in children with CLD or CHD who received BEYFORTUS (N=220) or palivizumab (N=42) in their second RSV season. The safety profile of BEYFORTUS in these children during their second RSV season was consistent with the safety profile of BEYFORTUS observed during their first RSV season.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of BEYFORTUS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity reactions [see Warnings and Precautions (5.1)].
7. Drug Interactions
7.1 Interference with RT-PCR or Rapid Antigen Detection RSV Diagnostic Assays
Nirsevimab-alip does not interfere with reverse transcriptase polymerase chain reaction (RT-PCR) or rapid antigen detection RSV diagnostic assays that employ commercially available antibodies targeting antigenic site I, II, or IV on the RSV fusion (F) protein. For immunological assay results which are negative when clinical observations are consistent with RSV infection, it is recommended to confirm using an RT-PCR-based assay.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of BEYFORTUS have been established for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. The safety and efficacy of BEYFORTUS for this indication and populations are discussed throughout the labeling.
Use of BEYFORTUS for this indication is supported by evidence from adequate and well-controlled studies in neonates and infants from birth up to 12 months of age with additional pharmacokinetic and safety data in children up to 24 months of age [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Use of BEYFORTUS in this population is supported by the following:
- -
- Trial 03, a randomized, double-blind, placebo-controlled multicenter trial for the prevention of MA RSV LRTI conducted in preterm infants born at GA greater than or equal to 29 weeks and less than 35 weeks entering their first RSV season;
- -
- Trial 04, a double-blind, placebo-controlled multicenter trial, for the prevention of MA RSV LRTI in term and late preterm infants GA greater than or equal to 35 weeks entering their first RSV season;
- -
- Trial 05, a Phase 2/3 randomized, double-blind, palivizumab-controlled multicenter trial in pediatric subjects born less than 35 weeks GA and infants with CLD of prematurity or hemodynamically significant CHD entering their first or second RSV season.
In addition, BEYFORTUS was evaluated in an open-label, uncontrolled, single-dose trial (Trial 08) in 100 infants and children who were less than or equal to 24 months of age, who received BEYFORTUS in their first or second RSV season, and who had a wide variety of underlying diseases or treatments resulting in immune compromise. The safety profile of BEYFORTUS administered in Trial 08 was consistent with the safety profile of other trials of BEYFORTUS in infants and children [see Clinical Pharmacology (12.3)].
The safety and effectiveness of BEYFORTUS have not been established in children older than 24 months of age.
10. Overdosage
There is limited experience of overdose with BEYFORTUS.
There is no specific treatment for an overdose with BEYFORTUS. In the event of an overdose, the individual should be monitored for the occurrence of adverse reactions and provided with symptomatic treatment as appropriate.
11. Beyfortus Description
Nirsevimab-alip, a respiratory syncytial virus F protein-directed fusion inhibitor, is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody produced in Chinese hamster ovary (CHO) cells by recombinant DNA technology. The molecular weight is approximately 146.3 kDa.
BEYFORTUS (nirsevimab-alip) injection is a sterile, preservative-free, clear to opalescent, colorless to yellow solution for intramuscular injection. It is supplied in a single-dose siliconized Luer lock Type I glass pre-filled syringe with a FluroTec coated plunger stopper.
Each 0.5 mL contains 50 mg nirsevimab-alip, arginine hydrochloride (8 mg), histidine (1.1 mg), L-histidine hydrochloride monohydrate (1.6 mg), polysorbate 80 (0.1 mg), sucrose (21 mg), and water for injection (USP). The pH is 6.0.
Each 1 mL contains 100 mg nirsevimab-alip, arginine hydrochloride (17 mg), histidine (2.2 mg), L-histidine hydrochloride monohydrate (3.3 mg), polysorbate 80 (0.2 mg), sucrose (41 mg), and water for injection (USP). The pH is 6.0.
12. Beyfortus - Clinical Pharmacology
12.1 Mechanism of Action
BEYFORTUS is a monoclonal antibody with anti-RSV activity [see Microbiology (12.4)].
12.2 Pharmacodynamics
There is a positive correlation between a serum nirsevimab-alip AUC (based on clearance at baseline) above 12.8 mg*day/mL and a lower incidence of medically attended RSV lower respiratory tract infection (MA RSV LRTI). Following IM administration of nirsevimab-alip in adults, RSV neutralizing antibody levels in serum were approximately 4 times higher than baseline at 8 hours after nirsevimab-alip dosing, and maximum levels were reached by day 6 following IM administration of nirsevimab-alip in adults. The safety and effectiveness of BEYFORTUS have not been established in adults.
Duration of Protection
Based on clinical data, the duration of protection offered by a single dose of BEYFORTUS extends through 5 months.
12.3 Pharmacokinetics
The PK of nirsevimab-alip is dose-proportional following a single IM administration of doses ranging from 25 mg (0.5 times the lowest approved recommended dosage) to 200 mg in pediatric subjects. Following the recommended dose, the nirsevimab-alip serum exposures were similar in neonates and infants born during or entering their first RSV season (Trials 03 and 04), and in neonates and infants born at less than 35 weeks GA (including less than 29 weeks GA) in their first RSV season (Trial 05), and in pediatric subjects up to 24 months of age with CLD or CHD in their first and second RSV season (Trial 05).
Absorption
The estimated nirsevimab-alip absolute bioavailability is 84% and the median time (range) to maximum concentration is 6 (1, 28) days.
Distribution
The estimated nirsevimab-alip total volume of distribution is 477 mL, for an infant weighing 5 kg.
Elimination
The nirsevimab-alip terminal half-life is approximately 71 days and the estimated clearance is 3.42 mL/day for an infant weighing 5 kg.
Metabolism
Nirsevimab-alip is degraded into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of nirsevimab-alip were observed based on race or vulnerability to severe RSV disease (i.e., CLD, CHD, GA <29 weeks).
The mean and median serum nirsevimab-alip concentrations in Trial 08 were lower than the concentrations in Trials 04 and 05. However, Trial 08 exposures were within the range shown to be effective in those who received the recommended dosage in Trials 03, 04, and 05 [see Pediatric Use (8.4)].
Drug Interaction Studies
No formal drug interaction studies have been performed with BEYFORTUS. Nirsevimab-alip is not predicted to be a substrate of, inhibitor or inducer of cytochrome P450 enzymes or transporter systems based on a mechanistic understanding of monoclonal antibodies.
Clinical Studies
Vaccines: There is limited experience with co-administration of BEYFORTUS with vaccines. In clinical trials, when BEYFORTUS was given concomitantly with routine childhood vaccines, the safety and reactogenicity profile of the co-administered regimen was similar to the childhood vaccines given alone.
12.4 Microbiology
Mechanism of Action
Nirsevimab-alip is a recombinant human IgG1κ monoclonal antibody that provides passive immunity by targeting the prefusion conformation of the RSV F protein. Nirsevimab-alip is long-acting due to a triple amino acid substitution (YTE) in the Fc region which increases binding to the neonatal Fc receptor and thereby extends serum half-life. Nirsevimab-alip binds to a conserved epitope in antigenic site Ø on the prefusion protein with dissociation constants KD = 0.12 nM and KD = 1.22 nM for RSV subtype A and B strains, respectively; it neutralizes RSV by inhibiting conformation changes in the F protein necessary for fusion of the viral and cellular membranes and viral entry.
Antiviral Activity
The cell culture neutralization activity of nirsevimab-alip against RSV was measured in a concentration-response model using cultured Hep-2 cells. Nirsevimab-alip neutralized clinical RSV isolates collected from global locations between 2003 and 2017 with median EC50 values for RSV A of 21 pM (3.2 ng/mL) (n=70; range 3 pM [0.48 ng/mL] to 100 pM [15 ng/mL]) and for RSV B of 19 pM (2.9 ng/mL) (n=49; range 2 pM [0.3 ng/mL] to 398 pM [59.7 ng/mL]).
Antiviral Resistance
In Cell Culture
Escape variants were selected following three passages in cell culture of RSV A2 and B9320 strains in the presence of nirsevimab-alip. Recombinant RSV A variants that showed reduced susceptibility to nirsevimab-alip compared with the reference strain included those with substitutions N67I+N208Y (103-fold reduction). Recombinant RSV B variants that showed reduced susceptibility to nirsevimab-alip included those with substitutions N208D (>90,000-fold change), N208S (>24,000-fold change), K68N+N201S (>13,000-fold change), and K68N+N208S (>90,000-fold change). All resistance-associated substitutions identified among neutralization escape variants were located in the nirsevimab-alip binding site (amino acids 62-69 and 196-212) and were shown to reduce binding affinity to RSV F protein.
In Surveillance Trials
Polymorphisms conferring large fold-reductions in susceptibility to nirsevimab-alip in isolates collected from 1956-2014 were not observed for RSV A and seen rarely (<1%) for RSV B, and included K65Q+K68N (1,239-fold change), K65Q+S211N (36-fold change), and L203I (3,005-fold change) substitutions. In prospective, observational, global molecular epidemiology studies (OUTSMART-RSV and INFORM-RSV) genetic diversity of RSV F protein sequences has remained low (most amino acids in both RSV A and RSV B >99% conserved). Variants harboring known nirsevimab-alip resistance-associated substitutions have been rare (<1%) and include RSV B substitutions K68Q (>369-fold change), N201T (>406-fold change) and N201T+I206M+Q209R (>418-fold change). Variants observed with reduced susceptibility include RSV A substitutions K68E (13-fold change), K68N (5-fold change), and S275F (6-fold change), and RSV B substitutions K68N (30-fold change), K68Q+I206M+Q209R (46-fold change), N201S (127-fold change) and N201S+I206M+Q209R (17-fold change). The clinical significance of these reductions in susceptibility is not known. From 2015 to 2021, most amino acid residues in the nirsevimab-alip binding site were highly conserved (>99%) at all positions in RSV A and 22 of the 25 positions in RSV B. Co-occurring substitutions I206M+Q209R in the nirsevimab-alip binding site that have become prevalent in RSV B since 2017 did not confer reduced susceptibility (<5-fold change) to nirsevimab-alip. The S211N substitution which has increased in prevalence also retains susceptibility to nirsevimab-alip, both individually and concurrently with I206M+Q209R.
In Clinical Trials
In Trial 04, Trial 05 and Trial 08 no known resistance-associated substitutions were identified at ≥25% frequency at any sampling time points. Phenotypic testing of novel substitutions is ongoing.
In Trial 03 (who received a single dose of 50 mg BEYFORTUS), 2 of 40 subjects with RSV infections corresponding to any case definition had a variant containing nirsevimab-alip resistance-associated substitutions. The two subjects received less than the recommended nirsevimab-alip dose and had RSV B variants harboring I64T+K68E+I206M+Q209R co-occurring substitutions or an N208S substitution. I64T, K68E, and N208S substitutions individually have reduced susceptibility to nirsevimab-alip (fold changes: >496, >283, and >387, respectively).
In Trial 04, an RSV B variant harboring binding site substitution L204S (no phenotypic data) concurrent with I206M+Q209R+S211N substitutions (<5-fold change) was detected at ≥25% frequency in one subject who received BEYFORTUS through Day 150. RSV B variants present at <25% frequency with I64T+K68E substitutions (>280-fold-change) and N208I substitution (>600-fold change) were seen in one subject each who received BEYFORTUS through Day 150. In addition, an RSV B N201D substitution (no phenotypic data) was observed at 9% frequency in one subject after Day 150.
Cross-resistance
Limited data are available that show that variants resistant to nirsevimab-alip could have cross-resistance to palivizumab. Palivizumab retained full neutralization potency against resistance-associated substitutions identified in Trial 03 and Trial 04. Nirsevimab-alip retained activity against recombinant RSV harboring palivizumab resistance-associated substitutions identified in molecular epidemiology studies and in neutralization escape variants of palivizumab; S275F substitution had reduced susceptibility of 6-fold.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of nirsevimab-alip or of other nirsevimab products.
Of 572 subjects receiving the recommended dose of nirsevimab-alip in Trial 03, 3.3% (16/492) subjects were ADA-positive on Day 361. Among the 16 ADA-positive subjects, 94% (15/16) had ADA against YTE and none tested positive for neutralizing antibodies against nirsevimab-alip.
In Trial 04, 5% (95/1778) of subjects were ADA-positive on Day 361, of whom 21% (20/95) had neutralizing antibodies and 77% (73/95) had ADA against YTE.
In Trial 05, among subjects enrolled during their first RSV season, on Day 361, 6% (32/538) of subjects were positive for ADA. Among the 32 ADA-positive subjects, 6% (2/32) had neutralizing antibodies against nirsevimab-alip and 91% (29/32) had antibodies against the YTE substitution. Of 180 subjects who received nirsevimab-alip in two consecutive RSV seasons, on season 2 Day 361, 9% (13/144) of subjects were positive for ADA. Among the 13 ADA-positive subjects, 8% (1/13) had neutralizing antibodies against nirsevimab-alip and 62% (8/13) had antibodies against YTE.
In Trial 08, among subjects receiving nirsevimab-alip in their first or second RSV season, 13% (9/67) of subjects were positive for ADA on Day 361. Among the 9 ADA-positive subjects, 11% (1/9) had neutralizing antibodies against nirsevimab-alip and 100% (9/9) were positive for ADA against YTE.
In Trials 03, 04, 05, and 08 the effect of ADA on nirsevimab-alip serum concentrations through Day 151 could not be determined. Subjects who received BEYFORTUS who developed anti-nirsevimab-alip antibodies had reduced nirsevimab-alip concentrations at Day 361 (40% to 60% lower compared to subjects who received BEYFORTUS who did not develop anti-nirsevimab-alip antibodies). Because of the low occurrence of ADA and MA RSV LRTI in clinical trials, the effect of these ADA on effectiveness of BEYFORTUS is unknown.
14. Clinical Studies
14.1 Description of Clinical Trials
The efficacy and safety of BEYFORTUS were evaluated in term and preterm infants and children in the trials summarized in Table 4.
|
||
|
Trial |
Population |
Study Arms |
|
D5290C00003 (Trial 03) NCT02878330 |
Infants born at ≥29 to <35 weeks GA entering their first RSV season |
BEYFORTUS (N=969)* Placebo (N=484) |
|
D5290C00004 (Trial 04) NCT03979313 |
Infants born at ≥35 weeks GA entering their first RSV season |
Primary Cohort†: BEYFORTUS (N=994) Placebo (N=496) Safety Cohort‡: BEYFORTUS (N=1,015) Placebo (N=507) |
|
D5290C00005 (Trial 05) NCT03959488 |
Infants born at <35 weeks GA and infants born with CLD or CHD entering their first RSV season Infants with CLD or CHD only entering their second RSV season |
RSV Season One: BEYFORTUS (N=616) Palivizumab (N=304) RSV Season Two: BEYFORTUS (N=220) Palivizumab (N=42) |
|
GA gestational age; CLD chronic lung disease; CHD hemodynamically significant chronic heart disease |
||
14.2 Prevention of MA RSV LRTI in Infants Born at ≥29 to <35 Weeks Gestational Age (Trial 03)
Trial 03 was a randomized, double-blind, placebo-controlled multicenter trial for the prevention of Medically Attended Respiratory Syncytial Virus Lower Respiratory Tract Infection (MA RSV LRTI) conducted in preterm infants born at gestational age (GA) greater than or equal to 29 weeks and less than 35 weeks. These subjects were randomized 2:1 to receive BEYFORTUS (N=969) or placebo (N=484) by IM injection. All subjects in the BEYFORTUS arm received 50 mg IM of BEYFORTUS regardless of body weight. The recommended BEYFORTUS dose in neonates and infants born during or entering their first RSV season is a single IM 50 mg or 100 mg dose for those who weigh less than 5 kg and greater than or equal to 5 kg, respectively [see Dosage and Administration (2.1)].
At randomization, 20% were GA greater than or equal to 29 weeks and less than 32 weeks; 80% were GA greater than or equal to 32 and less than 35 weeks; 52% were male; 72% were White; 18% were Black; 1% were Asian; 1% were Pacific Islander, and 8% were Other or Mixed Race; 22% were Hispanic or Latino; 68% were from Northern Hemisphere. The median age was 2.8 months (range: 0.1 to 11.9 months); 53% were less than or equal to 3 months; 33% were greater than 3 to less than or equal to 6 months, and 14% were greater than 6 months of age.
The primary endpoint was the incidence of MA RSV LRTI caused by RT-PCR-confirmed RSV, characterized predominantly as bronchiolitis or pneumonia through 150 days after dosing. Medically Attended (MA) includes all healthcare provider visits such as physician office, urgent care, emergency room visits and hospitalizations. Signs of LRTI involvement included rhonchi, rales, crackles, or wheezing; and at least one sign of worsening clinical severity including at least one of the following: increased respiratory rate, hypoxemia, acute hypoxic or ventilatory failure, new onset apnea, nasal flaring, retractions, grunting, or dehydration due to respiratory distress. Incidence of RSV LRTI with hospitalization was a prespecified secondary endpoint. RSV hospitalization was defined as hospitalization for LRTI with a positive RSV test.
Table 5 displays the primary efficacy result for Trial 03.
|
|||
|
N |
Incidence % (n) |
Efficacy* (95% CI) |
|
|
BEYFORTUS |
969 |
2.6% (25) | |
|
Placebo |
484 |
9.5% (46) |
|
In Trial 03, the efficacy of BEYFORTUS against MA RSV LRTI with hospitalization in infants born at GA greater than or equal to 29 weeks and less than 35 weeks, who received a single dose of 50 mg BEYFORTUS, based on the relative risk reduction was 78.4% (95% CI 51.9, 90.3; p=0.0002), through 150 days post dose.
14.3 Prevention of MA RSV LRTI in Infants Born at ≥35 Weeks Gestational Age (Trial 04)
BEYFORTUS was evaluated in one Phase 3 randomized, double-blind, placebo-controlled multicenter trial, Trial 04, for the prevention of MA RSV LRTI in term and late preterm infants GA greater than or equal to 35 weeks entering their first RSV season.
The primary analysis population (Primary Cohort) included 1,490 term and late preterm infants (GA greater than or equal to 35 weeks). Subjects were randomized 2:1 to receive a single IM dose of BEYFORTUS (N=994) (50 mg if less than 5 kg body weight or 100 mg if greater than or equal to 5 kg body weight at the time of dosing), or placebo (N=496). At randomization, 14% were GA greater than or equal to 35 weeks and less than 37 weeks; 86% were GA greater than or equal to 37 weeks; 52% were male; 53% were White; 28% were Black; 6% were American Indian/Alaskan native; 4% were Asian; 1% were Pacific Islander; and 8% were Other or Mixed Race; 10% were Hispanic or Latino; 69% were from Northern Hemisphere; and 40% weighed less than 5 kg. The median age was 2.6 months (range: 0.03 to 11.10 months); 58% were less than or equal to 3 months; 32% were greater than 3 to less than or equal to 6 months, and 10% were greater than 6 months of age.
In Trial 04, the primary endpoint was the incidence of MA RSV LRTI caused by RT-PCR-confirmed RSV, as defined in Trial 03. Incidence of RSV LRTI with hospitalization was a prespecified secondary endpoint. RSV hospitalization was defined as hospitalization for LRTI with a positive RSV test.
Table 6 displays the primary efficacy result from Trial 04.
|
N |
Incidence % (n) |
Efficacy† (95% CI) |
|
|
BEYFORTUS |
994 |
1.2% (12) |
74.9% (50.6, 87.3)‡ |
|
Placebo |
496 |
5.0% (25) |
|
In Trial 04, the efficacy of BEYFORTUS against MA RSV LRTI with hospitalization in infants born at GA greater than or equal to 35 weeks, who received a single IM 50 mg or 100 mg dose for those who weigh less than 5 kg and greater than or equal to 5 kg, respectively, based on the relative risk reduction was 60.2% (95% CI -14.6, 86.2; p=0.09), through 150 days post dose.
14.4 Prevention of MA RSV LRTI in Infants Born at <35 Weeks Gestational Age and Infants with CLD of Prematurity or Hemodynamically Significant CHD (Trial 05)
The safety and PK of BEYFORTUS were evaluated in a Phase 2/3 randomized, double-blind, palivizumab-controlled multicenter trial (Trial 05) in pediatric subjects born less than 35 weeks GA and infants with CLD of prematurity or hemodynamically significant CHD. This trial was not powered for efficacy, but efficacy was assessed as secondary endpoint. The efficacy of BEYFORTUS in preterm infants (GA less than 35 weeks) during their first RSV season and in pediatric subjects up to 24 months of age with CLD or CHD during their first and second RSV season was established by extrapolation of efficacy of BEYFORTUS from Trials 03 and Trial 04 to the population enrolled in Trial 05 based on similar nirsevimab-alip exposures among subjects enrolled in Trial 04 and 05 [see Clinical Pharmacology (12.3)].
Trial 05: RSV Season One
Trial 05 enrolled infants at higher risk for severe RSV disease entering their first RSV season into one of two cohorts: preterm infants (GA less than 35 weeks) and infants with CLD of prematurity or hemodynamically significant CHD. A total of 925 infants were randomized 2:1 in each of the preterm (n=615) and CLD/CHD (n=310) cohorts to receive BEYFORTUS or palivizumab. Infants received a single IM dose of BEYFORTUS (50 mg if less than 5 kg body weight or 100 mg if greater than 5 kg body weight at the time of dosing), followed by 4 once-monthly IM doses of placebo, or 5 once-monthly IM doses of 15 mg/kg palivizumab, respectively. At randomization, in the preterm cohort, 77 infants (13%) were less than 29 weeks GA; and 499 (81%) were GA greater than or equal to 29 to less than 35 weeks. In the CLD/CHD cohort, 70% had CLD of prematurity; 34% had hemodynamically significant CHD; 123 infants (40%) were less than 29 weeks GA, 28% were greater than or equal to 29 weeks to less than 35 weeks GA; and 32% were greater than or equal to 35 weeks GA. In both cohorts together, 54% were male; 79% were White; 10% were Black; 5% were Asian; 2% were American Indian/Alaskan Native; 15% were Hispanic or Latino; and 57% weighed less than 5 kg. The median age was 3.5 months (range: 0.07 to 12.3 months); 45% were less than or equal to 3 months; 34% were greater than 3 months to less than or equal to 6 months, and 21% were greater than 6 months of age.
In the first RSV season of Trial 05, the incidence of MA RSV LRTI through 150 days post dose was 0.6% (4/616) in the BEYFORTUS group and 1.0% (3/309) in the palivizumab group.
Trial 05: RSV Season Two
Pediatric subjects with CLD of prematurity or hemodynamically significant CHD up to 24 months of age continued in the trial for a second RSV season (n=262). Subjects who received BEYFORTUS during their first RSV season also received a single dose of 200 mg BEYFORTUS entering their second RSV season followed by 4 once-monthly IM doses of placebo (n=180). Subjects who received palivizumab during their first RSV season were re-randomized 1:1 to either receive BEYFORTUS or palivizumab entering their second RSV season. Forty subjects who received palivizumab in the first RSV season received a single IM dose of BEYFORTUS followed by 4 once-monthly IM doses of placebo in their second RSV season; and 42 subjects received palivizumab (5 once-monthly IM doses of 15 mg/kg palivizumab) in both first and second RSV seasons.
In the second RSV season of Trial 05, there were no cases of MA RSV LRTI through Day 150 post-dose in subjects who received either BEYFORTUS or palivizumab.
16. How is Beyfortus supplied
How Supplied
BEYFORTUS injection is a sterile, preservative-free, clear to opalescent, colorless to yellow solution supplied as follows:
- •
- One 50 mg/0.5 mL single-dose pre-filled syringe in a carton: NDC 49281-575-00
- •
- Five 50 mg/0.5 mL single-dose pre-filled syringes in a carton: NDC 49281-575-15
- •
- One 100 mg/mL single-dose pre-filled syringe in a carton: NDC 49281-574-88
- •
- Five 100 mg/mL single-dose pre-filled syringes in a carton: NDC 49281-574-15
Each BEYFORTUS pre-filled syringe is for one time use only.
Storage and Handling
Store refrigerated between 36°F to 46°F (2°C to 8°C). BEYFORTUS may be kept at room temperature 68°F to 77°F (20°C to 25°C) for a maximum of 8 hours. After removal from the refrigerator, BEYFORTUS must be used within 8 hours or discarded.
Store BEYFORTUS in original carton to protect from light until time of use.
Do not freeze. Do not shake. Do not expose to heat.
17. Patient Counseling Information
Advise the child’s caregiver to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions Including Anaphylaxis
Inform the patient’s caregiver of the signs and symptoms of potential hypersensitivity reactions, and advise the caregiver to seek medical attention immediately if the child experiences a hypersensitivity reaction to BEYFORTUS [see Warnings and Precautions (5.1)].
Dosage and Administration
Advise the caregiver that the child will receive one dose of BEYFORTUS by IM injection by a healthcare provider. If the child remains at increased risk for RSV, they may receive a second dose in the second RSV season [see Dosage and Administration (2.1)].
Manufactured by: AstraZeneca AB, Södertälje, Sweden SE-15185
US License No. 2059
Distributed by: Sanofi Pasteur, Inc., Swiftwater, PA 18370 USA
BEYFORTUS is a trademark of the Sanofi group of companies.
©AstraZeneca 2024
|
PATIENT INFORMATION BEYFORTUS™ (Bay for tus) (nirsevimab-alip) injection, for intramuscular use |
|
|
What is BEYFORTUS? BEYFORTUS is a prescription medicine that is used to help prevent a serious lung disease caused by Respiratory Syncytial Virus (RSV) in:
BEYFORTUS is an antibody that contains nirsevimab-alip which is used to help prevent RSV disease for 5 months. It is not known if BEYFORTUS is safe and effective in children older than 24 months of age. |
|
|
Your child should not receive BEYFORTUS if your child has a history of serious allergic reactions to nirsevimab-alip or any of the ingredients in BEYFORTUS. See the end of this Patient Information leaflet for a complete list of ingredients in BEYFORTUS. |
|
|
Before your child receives BEYFORTUS, tell your healthcare provider about all of your child’s medical conditions, including if your child:
Tell your child’s healthcare provider about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Your infant should not receive a medicine called palivizumab if they have already received BEYFORTUS in the same RSV season. |
|
|
How is BEYFORTUS given?
|
|
|
What are the possible side effects of BEYFORTUS?
|
|
|
|
|
The most common side effects of BEYFORTUS include rash, and pain, swelling or hardness at the site of your child’s injection. These are not all of the possible side effects of BEYFORTUS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
General information about the safe and effective use of BEYFORTUS. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about BEYFORTUS that is written for health professionals. |
|
|
What are the ingredients in BEYFORTUS? Active ingredient: nirsevimab-alip Inactive ingredients: arginine hydrochloride, histidine, L-histidine hydrochloride monohydrate, polysorbate 80, sucrose and water for injection. |
|
|
Manufactured by: AstraZeneca AB, Södertälje, Sweden SE-15185 US License No. 2059 Distributed by: Sanofi Pasteur, Inc., Swiftwater, PA 18370 USA BEYFORTUS is a trademark of the Sanofi group of companies. ©AstraZeneca 2024 For more information, go to https://www.Beyfortus.com or call 1-855-239-3678 (1-855-BEYFORTUS). |
|
|
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: February 2024 |
|
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 49281-575-00 Rx only
Beyfortus™ (nirsevimab-alip) Injection 50mg/0.5mL
One 0.5 mL single-dose prefilled syringe
For Intramuscular Injection Only
Store the pre-filled syringe in original carton to protect from light until time of use.
Must be administered by a healthcare provider.
sanofi
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 49281-574-88 Rx only
Beyfortus™ (nirsevimab-alip) Injection 100mg/mL
One single-dose pre-filled syringe
For Intramuscular Injection Only
Store the pre-filled syringe in original carton to protect from light until time of use.
Must be administered by a healthcare provider
sanofi
| BEYFORTUS
nirsevimab injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BEYFORTUS
nirsevimab injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Registrant - AstraZeneca PLC (230790719) |
Biological Products Related to Beyfortus
Find detailed information on biosimilars for this medication.
Frequently asked questions
More about Beyfortus (nirsevimab)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antiviral monoclonal antibodies
- En español