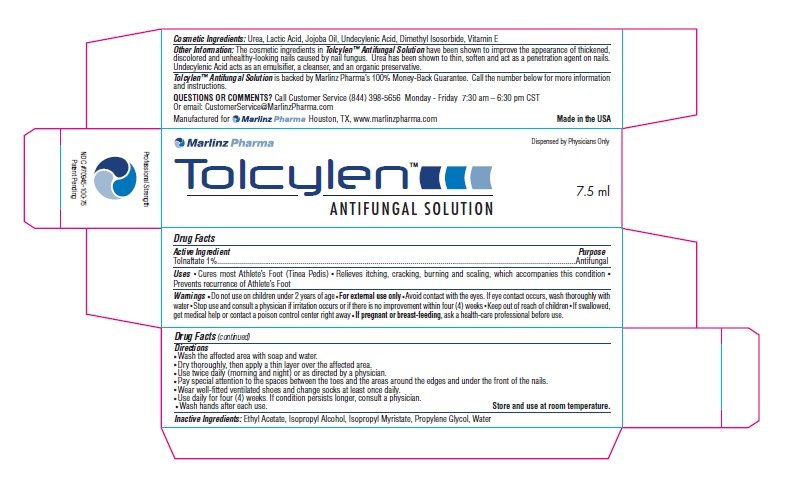
Tolcylen Antifungal Solution
Dosage form: solution
Ingredients: TOLNAFTATE 1mg in 100mL
Labeler: Marlinz Pharma, LLC
NDC code: 70945-100
Medically reviewed by Drugs.com. Last updated on Jun 3, 2024.
Tolnaftate 1%
Antifungal
Keep out of reach of children
Cures most Athlete’s Foot (Tinea Pedis) ■ Relieves itching, cracking, burning and scaling, which accompanies this condition ■
Prevents recurrence of Athlete’s Foot
Do not use on children under 2 years of age ■ For external use only ■ Avoid contact with the eyes. If eye contact occurs, wash thoroughly with
water ■ Stop use and consult a physician if irritation occurs or if there is no improvement within four (4) weeks ■ Keep out of reach of children ■ If swallowed,
get medical help or contact a poison control center right away ■ If pregnant or breast-feeding, ask a health-care professional before use.
Wash the affected area with soap and water.
■ Dry thoroughly, then apply a thin layer over the affected area.
■ Use twice daily (morning and night) or as directed by a physician.
■ Pay special attention to the spaces between the toes and the areas around the edges and under the front of the nails.
■ Wear well-fitted ventilated shoes and change socks at least once daily.
■ Use daily for four (4) weeks. If condition persists longer, consult a physician.
■ Wash hands after each use. Store and use at room temperature.
Inactive Ingredients: Ethyl Acetate, Isopropyl Alcohol, Isopropyl Myristate, Propylene Glycol, Water
Cosmetic Ingredients: Urea, Lactic Acid, Jojoba Oil, Undecylenic Acid, Dimethyl Isosorbide, Vitamin E
| TOLCYLEN ANTIFUNGAL SOLUTION
tolnaftate solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Marlinz Pharma, LLC (080251509) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Marlinz Pharma, LLC | 080251509 | manufacture(70945-100) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.