Assured Allergy Relief
Dosage form: tablet
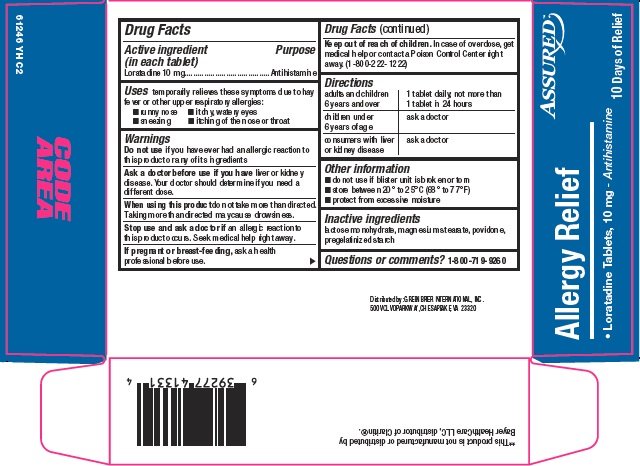
Ingredients: LORATADINE 10mg
Labeler: Greenbrier International Inc.
NDC code: 33992-0612
Medically reviewed by Drugs.com. Last updated on Feb 8, 2024.
Loratadine 10 mg
Antihistamine
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
if you have ever had an allergic reaction to this product or any of its ingredients
liver or kidney disease. Your doctor should determine if you need a different dose.
do not take more than directed. Taking more than directed may cause drowsiness.
an allergic reaction to this product occurs. Seek medical help right away.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
- •
- do not use if blister unit is broken or torn
- •
- store between 20° to 25°C (68° to 77°F)
- •
- protect from excessive moisture
lactose monohydrate, magnesium stearate, povidone, pregelatinized starch
1-800-719-9260
COMPARE TO ACTIVE INGREDIENT OF CLARITIN®
24 HOUR
Allergy Relief
Loratadine Tablets, 10 mg – Antihistamine
Indoor & Outdoor Allergies
24 Hour Relief of: Sneezing; Runny Nose;
Itchy, Watery Eyes; Itchy Throat or Nose
Non-Drowsy*
Original Prescription Strength
*When taken as directed. See Drug Facts Panel.
Actual Size
10 Days of Relief
10 tablets
| ASSURED ALLERGY RELIEF
loratadine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Greenbrier International Inc. (610322518) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.