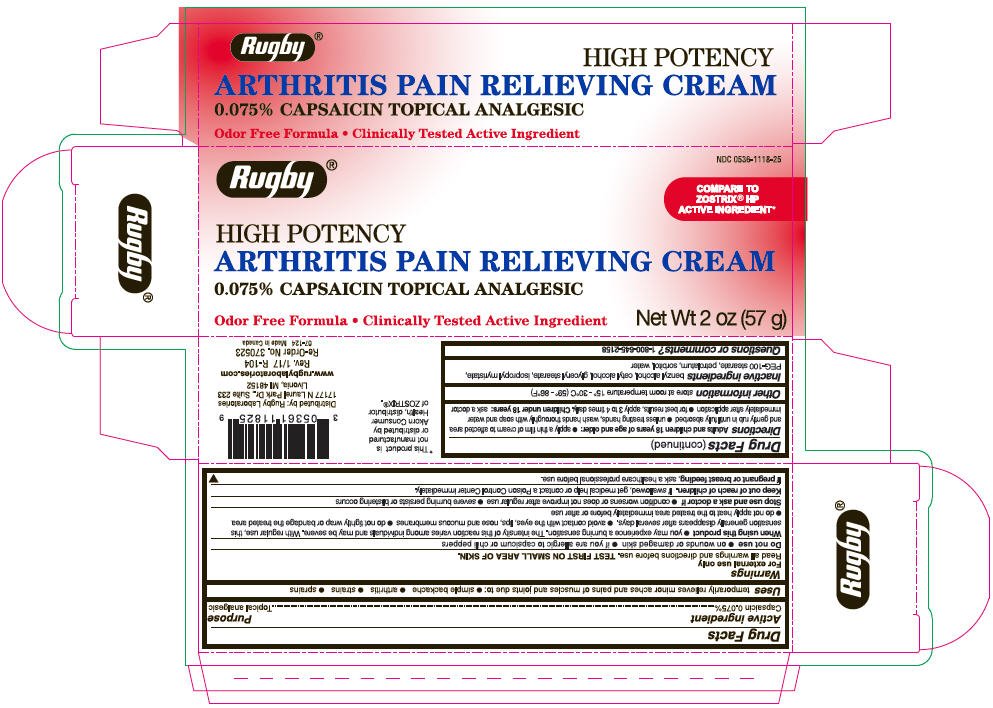
Rugby Arthritis Pain Relieving Topical Analgesic
Dosage form: cream
Ingredients: Capsaicin 0.75mg in 1g
Labeler: Rugby Laboratories
NDC code: 0536-1118
Medically reviewed by Drugs.com. Last updated on May 16, 2024.
Drug Facts
Capsaicin 0.075%
Topical Analgesic
temporarily relieves minor aches and pains of muscles and joints due to:
- ♦
- simple backache
- ♦
- arthritis
- ♦
- strains
- ♦
- sprains
For external use only
Read all warnings and directions before use. TEST FIRST ON SMALL AREA OF SKIN.
- ♦
- on wounds or damaged skin
- ♦
- if you are allergic to capsicum or chili peppers
- ♦
- you may experience a burning sensation. The intensity of this reaction varies among individuals and may be severe. With regular use, this sensation generally disappears after several days.
- ♦
- avoid contact with eyes, lips, nose and mucous membranes
- ♦
- do not tightly wrap or bandage the treated area
- ♦
- do not apply heat to the treated area immediately before or after use
- ♦
- condition worsens or does not improve after regular use
- ♦
- severe burning persists or blistering occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
If pregnant or breast feeding, ask a health professional before use.
Adults and children 18 years of age and older:
- ♦
- apply a thin film of cream to affected area and gently rub in until fully absorbed
- ♦
- unless treating hands, wash hands thoroughly with soap and water immediately after application
- ♦
- for best results, apply 3 to 4 times daily.
Children under 18 years: ask a doctor
Store at room temperature 15° - 30°C (59°F – 86°F).
benzyl alcohol, cetyl alcohol, glyceryl stearate, isopropyl myristate, PEG-100 stearate, petrolatum, sorbitol, water
1-800-645-2158
Distributed by: Rugby Laboratories
17177 N Laurel Park Dr., Suite 233
Livonia, MI 48152
| RUGBY ARTHRITIS PAIN RELIEVING
TOPICAL ANALGESIC
capsaicin cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Registrant - Garcoa, Inc (036464697) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Garcoa, Inc | 036464697 | MANUFACTURE(0536-1118) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.