CVS Povidone-Iodine
Dosage form: liquid
Ingredients: POVIDONE-IODINE 100mg in 1mL
Labeler: CVS Pharmacy
NDC code: 69842-325
Medically reviewed by Drugs.com. Last updated on Jun 12, 2024.
CVS Povidone-Iodine
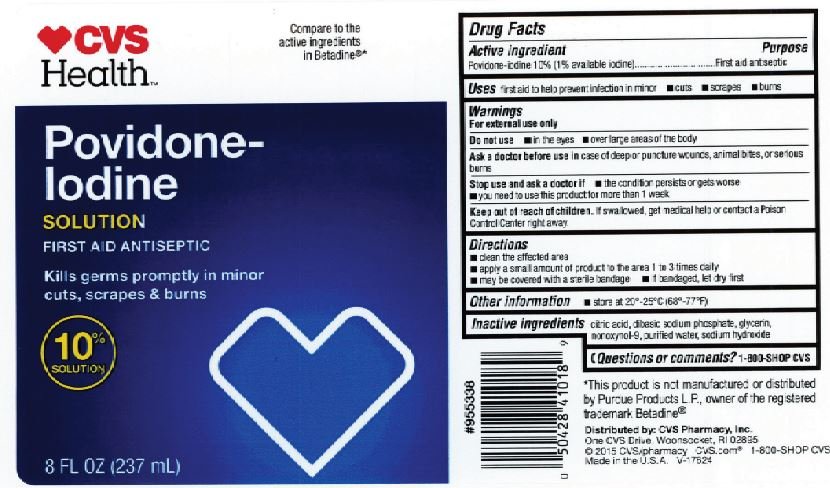
Drug Facts
Povidone-Iodine 10% (1% Available iodine)
First Aid Antiseptic
First aid to help prevent infection in in minor: cuts, scrapes and burns.
For external use only
in the eyes. Over large areas of the body.
in case of deep or puncture wounds, animal bites, or serious burns.
The condition persists or gets worse. Need to use this product for more than 1 week.
- If swallowed, get medical help or contact a Poison Control Center right away.
- Clean the affected area. Apply a small amount to the affected area 1 to 3 times daily. May be covered with a sterile bandage. If bandaged, let dry first.
Citric acid, Dibasic sodium Phosphate, Glycerin, Nonoxynol-9, Purified water, sodium hydroxide.
| CVS POVIDONE-IODINE
povidone iodine liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Humco Holding Group, Inc. (825672884) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Humco Holding Group, Inc. | 825672884 | analysis(69842-325), manufacture(69842-325), pack(69842-325), label(69842-325) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.