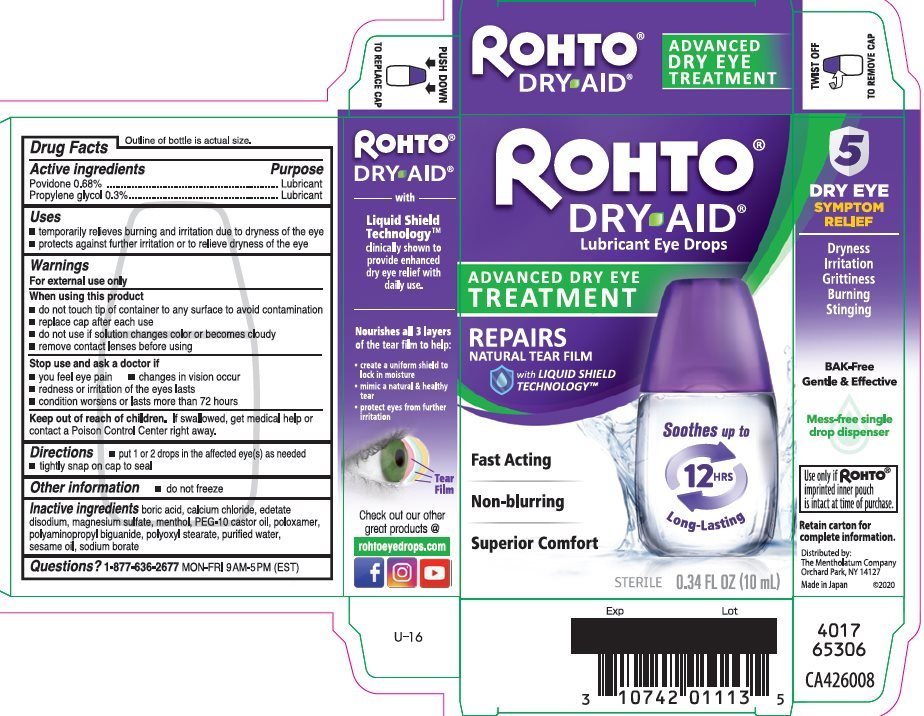
Rohto Dry-Aid
Dosage form: liquid
Ingredients: POVIDONE, UNSPECIFIED 6.8mg in 1mL, PROPYLENE GLYCOL 3mg in 1mL
Labeler: The Mentholatum Company
NDC code: 10742-8162
Medically reviewed by Drugs.com. Last updated on Feb 28, 2025.
Povidone 0.68%
Propylene glycol 0.3%
Povidone – Lubricant
Propylene glycol - Lubricant
- •
- temporarily relieves burning and irritation due to dryness of the eye
- •
- protects against further irritation or to relieve dryness of the eye
For external use only
- •
- do not touch tip of container to any surface to avoid contamination
- •
- replace cap after each use
- •
- do not use if solution changes color or becomes cloudy
- •
- remove contact lenses before using
- •
- you feel eye pain
- •
- changes in vision occur
- •
- redness or irritation of the eyes lasts
- •
- condition worsens or lasts more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away.
- •
- put 1 or 2 drops in the affected eye(s) as needed
- •
- tightly snap on cap to seal
Other information
- •
- do not freeze
boric acid, calcium chloride, edetate disodium, magnesium sulfate, menthol, PEG-10 castor oil, poloxamer, polyaminopropyl biguanide, polyoxyl stearate, purified water, sesame oil, sodium borate
1-877-636-2677 MON-FRI 9AM-5PM (EST)
| ROHTO DRY-AID
povidone, propylene glycol liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - The Mentholatum Company (002105757) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.