Equate Allergy Relief by Wal-Mart Stores, Inc.
Dosage form: tablet, film coated
Ingredients: cetirizine hydrochloride 10mg
Labeler: Wal-Mart Stores, Inc.
NDC code: 49035-312
Medically reviewed by Drugs.com. Last updated on Jul 24, 2024.
Cetirizine HCl 10 mg
Antihistamine
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat or nose
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
an allergic reaction to this product occurs. Seek medical help right away.
- if breast-feeding: not recommended
- if pregnant: ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
| adults and children 6 years and over | one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. |
| adults 65 years and over | ask a doctor |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
- do not use if printed foil under cap broken or missing
- store between 20° to 25°C (68° to 77°F)
Colloidal silicon dioxide, Hypromellose, lactose monohydrate, low-substituted hydroxyporpyl cellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol and titanium dioxide.
1-888-287-1915
NDC 49035-312-56
equate™
Allergy Relief
Cetirizine Hydrochloride Tablets USP, 10 mg
Antihistamine
Indoor and Outdoor Allergies
24 Hour Relief of:
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy throat or nose
24
HOUR
SYMPTOM
RELIEF
Original Prescription Strength
10 mg
14 TABLETS
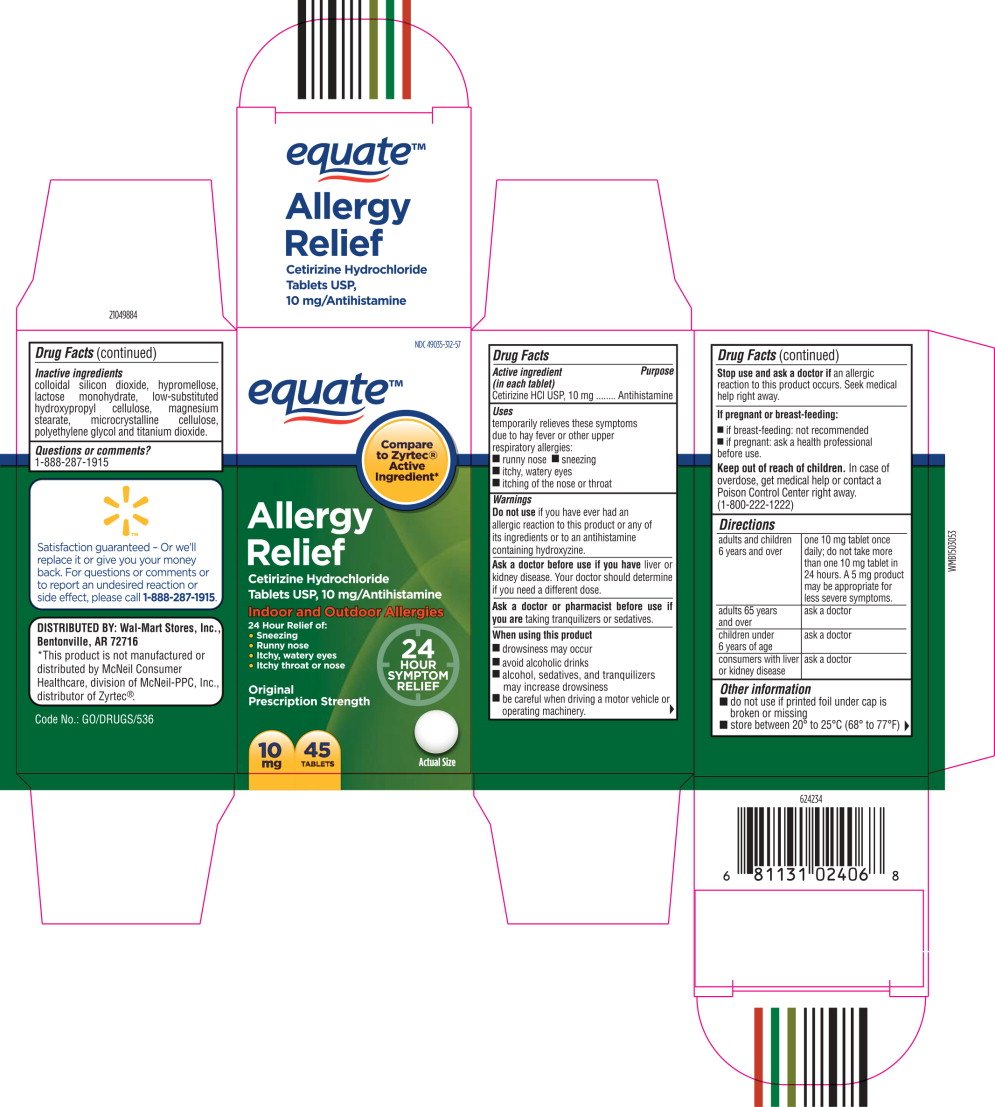
NDC 49035-312-57
equate™
Allergy
Relief
Cetirizine Hydrochloride
Tablets USP, 10 mg/Antihistamine
Indoor and Outdoor Allergies
24 Hour Relief of:
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy throat or nose
24
HOUR
SYMPTOM
RELIEF
Original
Prescription Strength
10 mg
45 TABLETS
equate™
NDC 49035-312-57
Allergy Relief
Cetirizine Hydrochloride
Tablets USP, 10 mg
Antihistamine
Original
Prescription
Strength
45 Tablets
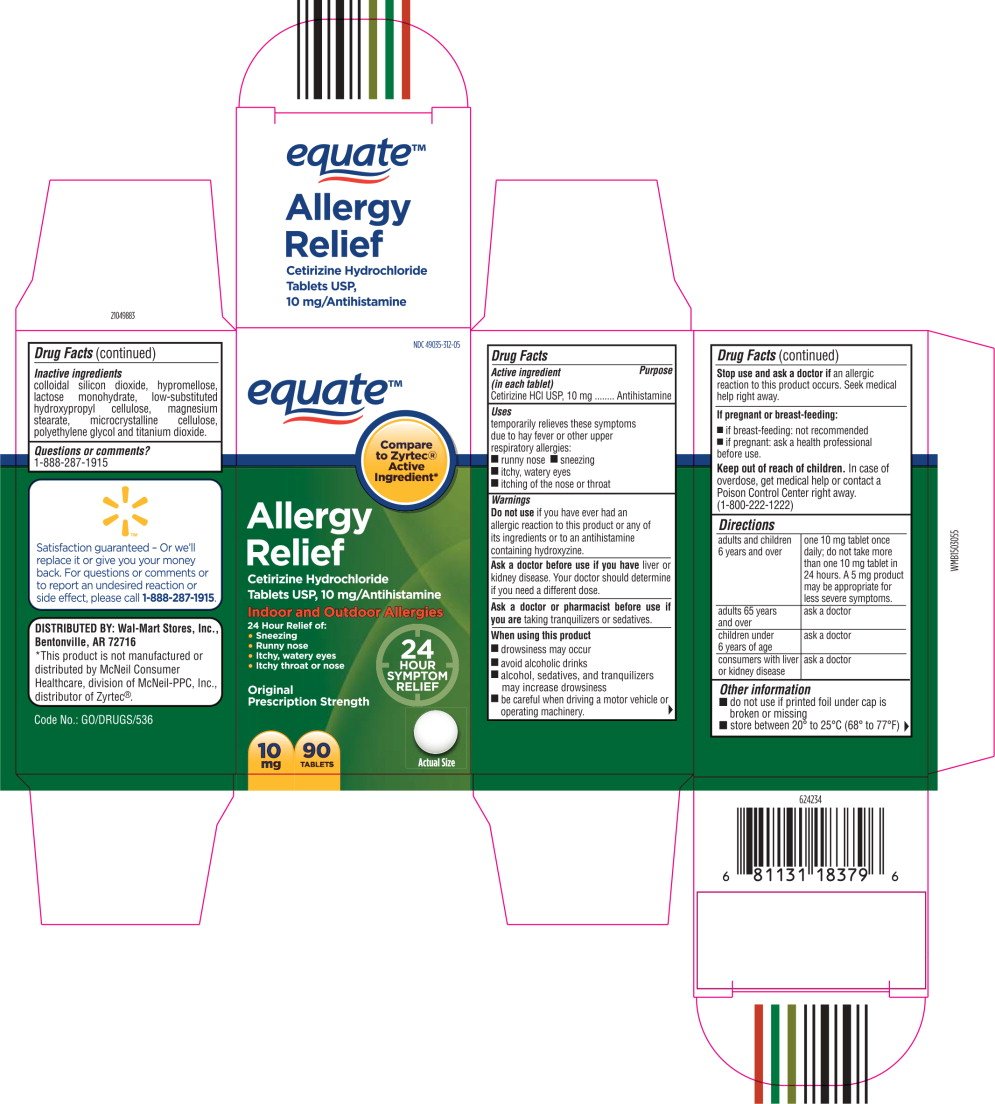
NDC 49035-312-05
equate™
Allergy
Relief
Cetirizine Hydrochloride
Tablets USP, 10 mg/Antihistamine
Indoor and Outdoor Allergies
24 Hour Relief of:
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy throat or nose
24
HOUR
SYMPTOM
RELIEF
Original
Prescription Strength
10 mg
90 TABLETS
equate™
NDC 49035-312-05
Allergy Relief
Cetirizine Hydrochloride
Tablets USP, 10 mg
Antihistamine
Original
Prescription
Strength
90 Tablets
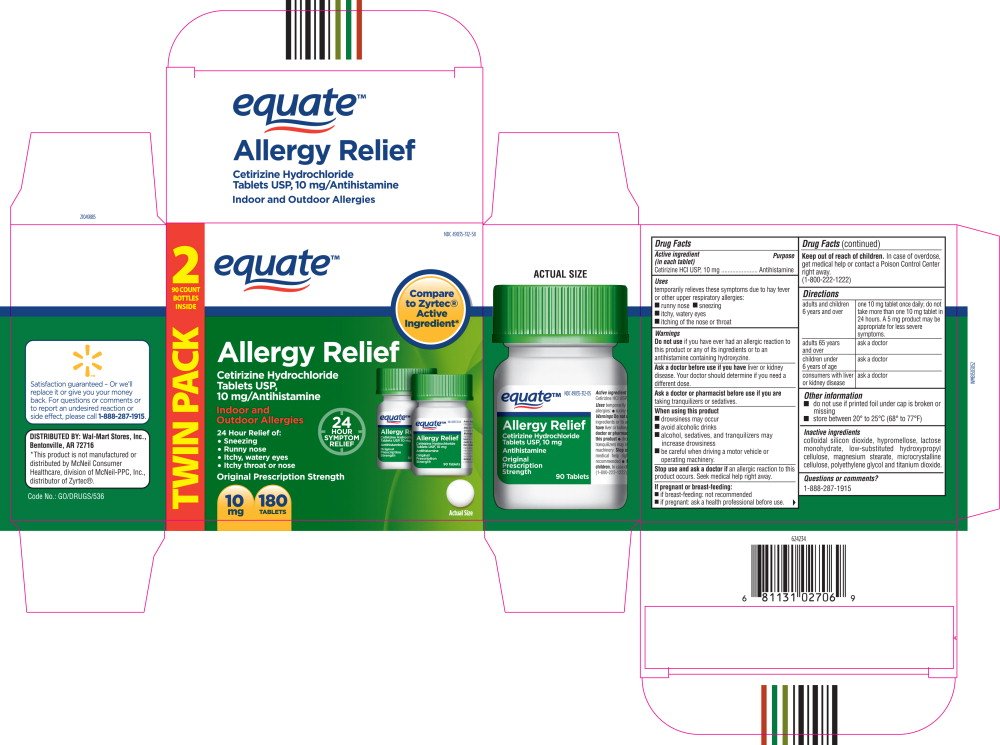
NDC 49035-312-58
equate™
Allergy Relief
Cetirizine Hydrochloride
Tablets USP,
10 mg/Antihistamine
Indoor and
Outdoor Allergies
24 Hour Relief of:
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy throat or nose
24
HOUR
SYMPTOM
RELIEF
Original Prescription Strength
10 mg
180 TABLETS
| EQUATE ALLERGY RELIEF
cetirizine hydrochloride tablet, film coated |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Labeler - Wal-Mart Stores, Inc. (051957769) |
| Registrant - Cipla Ltd. (650072015) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cipla limited | 650072015 | MANUFACTURE(49035-312) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cipla limited | 916940208 | API MANUFACTURE(49035-312) | |
See all Equate Allergy Relief brands
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.