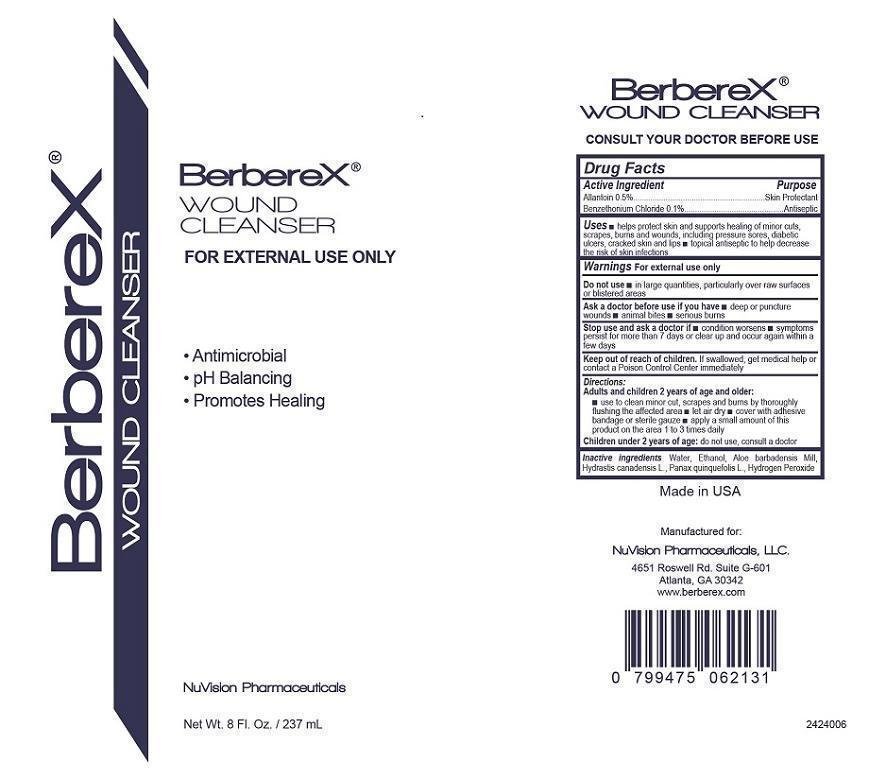
BerbereX Wound Cleanser
Dosage form: liquid
Ingredients: ALLANTOIN 5g in 1000mL, BENZETHONIUM CHLORIDE 1g in 1000mL
Labeler: Cosco International, Inc.
NDC code: 52261-0500
Medically reviewed by Drugs.com. Last updated on Jan 17, 2024.
Allantoin 0.5%........................Skin Protectant
Benzethonium Chloride............Antiseptic
- helps protect skin and supports healing of minor cuts, scrapes, burns and wounds, including pressure sores, diabetic ulcers, cracked skin and lips
- topical antiseptic to help decrease the risk of skin infections
Warnings For external use only.
- in large quantities, particularly over raw surfaces or blistered areas
- deep or puncture wounds
- animal bites
- serious burns
- condition worsens
- symptons persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately
Adults and children 2 years of age and older:
- use to clean minor cut, scrapes, and burns by thoroughly flushing the affected area
- let air dry
- cover with adhesive bandage or sterile gauze
- apply a small amount of this product on the area 1 to 3 times daily
Children under 2 years of age: do not use, consult a doctor
Aloe barbadensis Mill, Ethanol, Hydrastis canadensis L., Hydrogen Peroxide, Panax quinquefolis L., Water
| BERBEREX WOUND CLEANSER
benzethonium chloride liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Cosco International, Inc. (016433141) |
| Registrant - Cosco International, Inc. (016433141) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cosco International, Inc. | 016433141 | manufacture(52261-0500), label(52261-0500), pack(52261-0500) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.