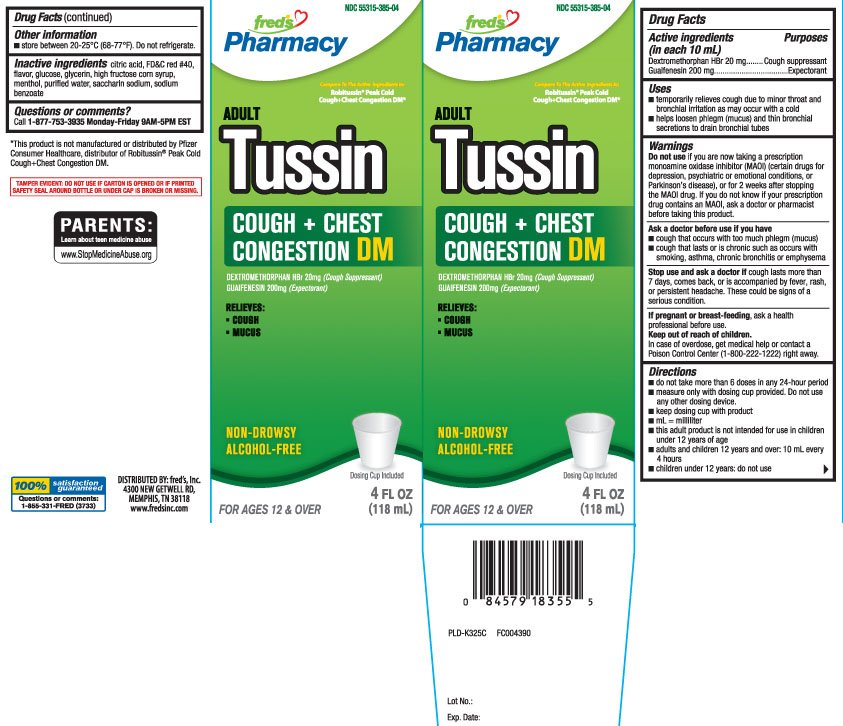
Adult Tussin Cough and Chest Congestion DM
Dosage form: liquid
Ingredients: DEXTROMETHORPHAN HYDROBROMIDE 20mg in 10mL, GUAIFENESIN 200mg in 10mL
Labeler: Freds Inc
NDC code: 55315-385
Medically reviewed by Drugs.com. Last updated on Dec 9, 2024.
Dextromethorphan HBr 20 mg
Guaifenesin 200 mg
Cough suppressant
Expectorant
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- do not take more than 6 doses in any 24-hour period
- measure only with the dosing cup provided. Do not use any other dosing device.
- keep dosing cup with product
- mL= milliliter
- this adult product is not intended for use in children under 12 years of age
- adults and children 12 years and over: 10 mL every 4 hours
- children under 12 years: do not use
- store between 20-25ºC(68-77ºF). Do not refrigerate
anhydrous citric acid, FD&C red 40, flavor, glucose, glycerin, high fructose corn syrup, menthol, purified water, saccharin sodium, sodium benzoate
Call 1-877-753-3935 Monday-Friday 9AM-5PM EST
Compare To The Active Ingredients in Pobitussin® Peak Cold Cpough-+Chest Congestion DM*
Adult
Tussin Cough + Chest Congestion DM
DEXTROMETHORPHAN HBr 20 mg (Cough Suppressant)
GUAIFENESIN 200 mg (Expectorant)
Relieves:
- Cough
- Mucus
NON-DROWSY
ALCOHOL-FREE
FL OZ (mL)
DOSAGE CUP INCLUDED
FOR AGES 12 & OVER
*This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of Robitussin® Peak Cold Cough + Chest Congestion DM.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
DISTRIBUTED BY: fred's, Inc.
4300 NEW GETWELL RD,
MEMPHIS, TN 38118
www.fredsinc.com
| ADULT TUSSIN COUGH AND CHEST CONGESTION DM
dextromethorphan hbr, guaifenesin liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Freds Inc (005866116) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.