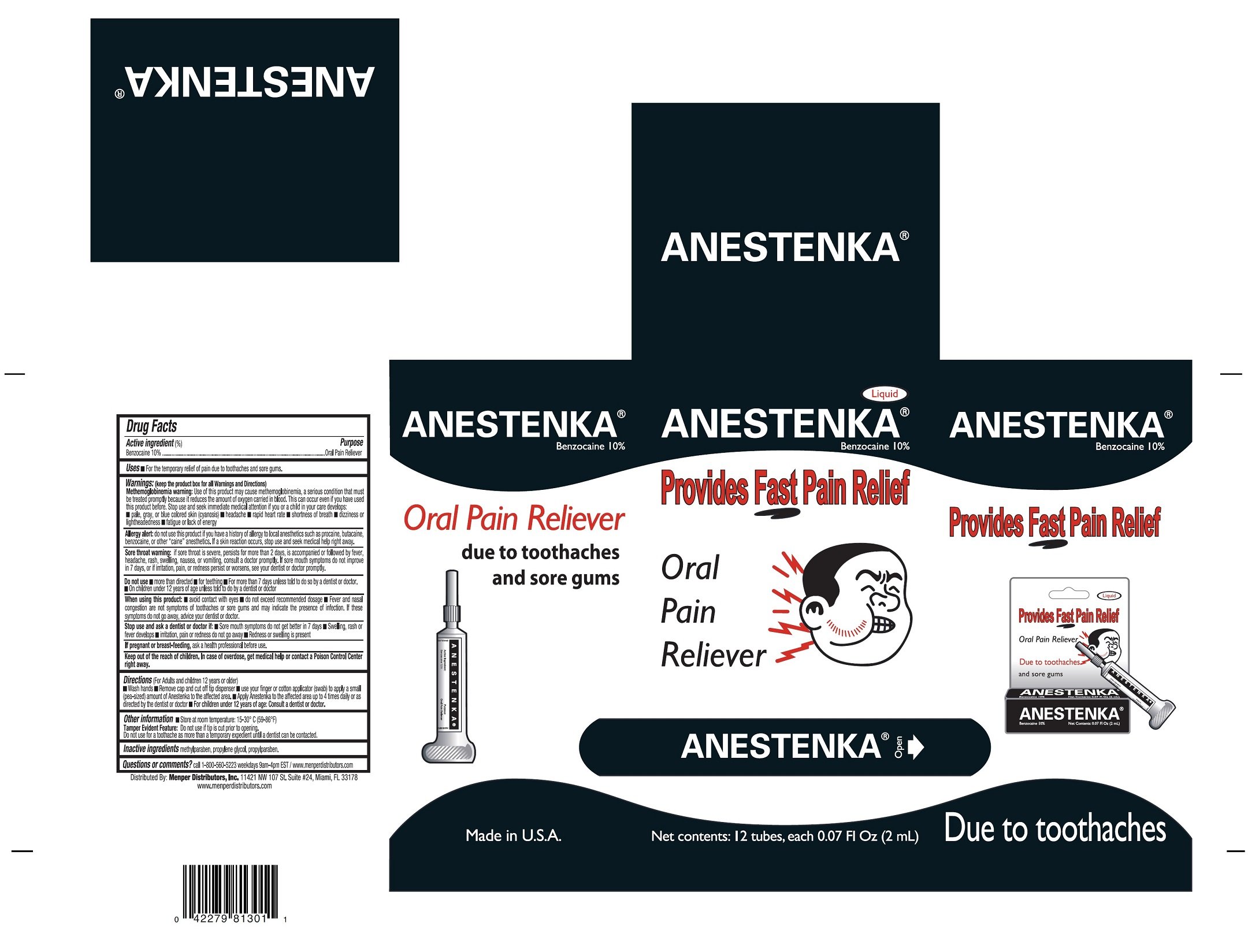
Anestenka
Dosage form: liquid
Ingredients: BENZOCAINE 0.2mg in 2mL
Labeler: Menper Distributors, Inc
NDC code: 53145-301
Medically reviewed by Drugs.com. Last updated on Jan 15, 2024.
active Ingrediets(%)
Benzocaine 10%
Oral Pain Reliever
For temporary relief of pain due to toothaches and sure gums.
Use of this product may cause methemoglobinemia, a seious condition that must be treated promtly because it reduces the amount of oxygen carried in blood. This can occurred even if you have used this product before. Stop used and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid hear rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
do not use this product if you have a history of allergy to local anesthethics such as procaine, butacaine, benzocaine, or other "caine" anesthetic. If skin reacction ocurrs, stop use and seek medical help right away.
if sore throat is severe, persists for more that two days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persist or worsens, see your dentist dentist or doctor promptly.
- more than directed
- for teething
- for more than 7 days unless told to do so by a dentist or doctor
- on child under 12 years of age unless told to do so by a dentist or doctor
- Avoid contact with eyes
- do not exceed recomended dosage
- fever and nasal congestion are not symptoms of toothache or sore gums and may indicate the presence of infection. If these symptoms do not go away, advise your dentist or doctor.
- Sore mouth symptoms do not get better in 7 days
- swelling, rash or fever develops
- irritation, pain, or redness do not go away
- Redness or swelling is presented
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
(For adults and children 12 years or older)
- wash hands
- Remove cap and cut off tip dispenser
- use your finger or cotton applicator (swab) to apply a small (pea-size) amount of Anestenka to the affected area
- Apply Anestenka to the affected area up to 4 times daily or as directed by the demtist or doctor
- For children 12 years of age: Consult a dentist or doctor
Store at room temperature: 15-30° C (59-86° F)
Tamper Evident Feature:
Do not use if tip is cut prior to opening.
Do not use for toothache as more than a temporary expedient until a dentidst can be contacted.
methylparaben, propylene glycol, propyparaben
call 1-800-560-5223 weekdays 9am-4pm EST/www.menperdistributors.com
| ANESTENKA
benzocaine liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Menper Distributors, Inc (101947166) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.