BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER
Dosage form: kit
Ingredients: ALCOHOL 75mL in 100mL
Labeler: ENCHANTE ACCESSORIES INC.
NDC code: 50563-210
Medically reviewed by Drugs.com. Last updated on May 27, 2024.
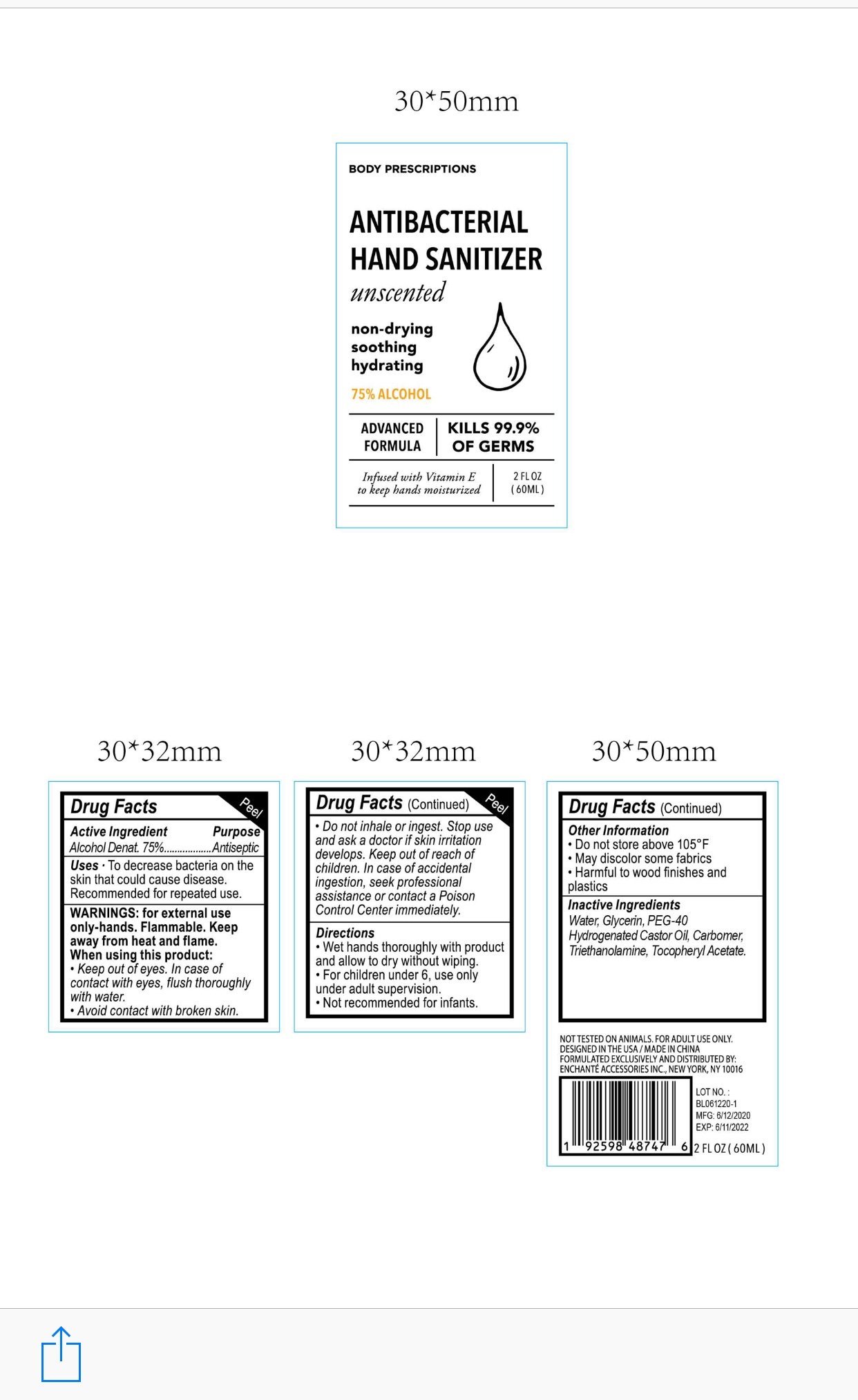
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER UNSCENTED
Alcohol Denat. 75%
Antiseptic
■ To decrease bacteria on the skin that could cause disease
■Recommended for repeated use
For external use only, Hands
Flammable. Keep away from heat and flame
When using this product, keep out of eyes.
In case of contact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest
Stop use and ask a doctor if skin irritation develops
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately
Wet hands thoroughly with product and allow to dry without wiping
For children under 6, use only under adult supervision
Not recommended for infants
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Water, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Tocopheryl Acetate
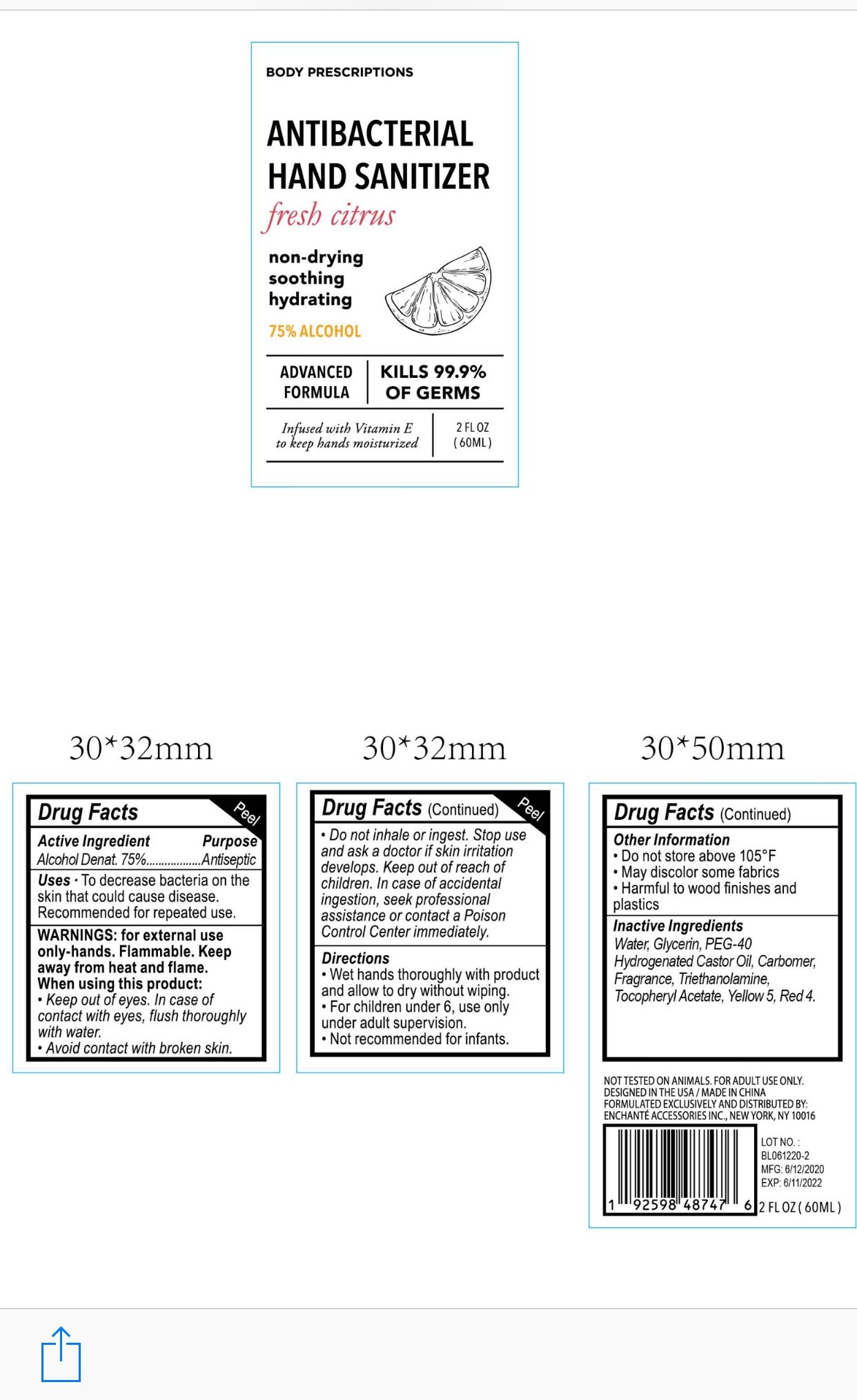
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER FRESH CITRUS
Active Ingredient
Alcohol Denat. 75%
Purpose:
Antiseptic
Uses:
To decrease bacteria on the skin that could cause disease
■Recommended for repeated use
For external use only, Hands
Flammable. Keep away from heat and flame
When using this product, keep out of eyes.
In case of contact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest
Stop use and ask a doctor if skin irritation develops
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately
Wet hands thoroughly with product and allow to dry without wiping
For children under 6, use only under adult supervision
Not recommended for infants
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Water, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Tocopheryl Acetate, Yellow 5, Red 4
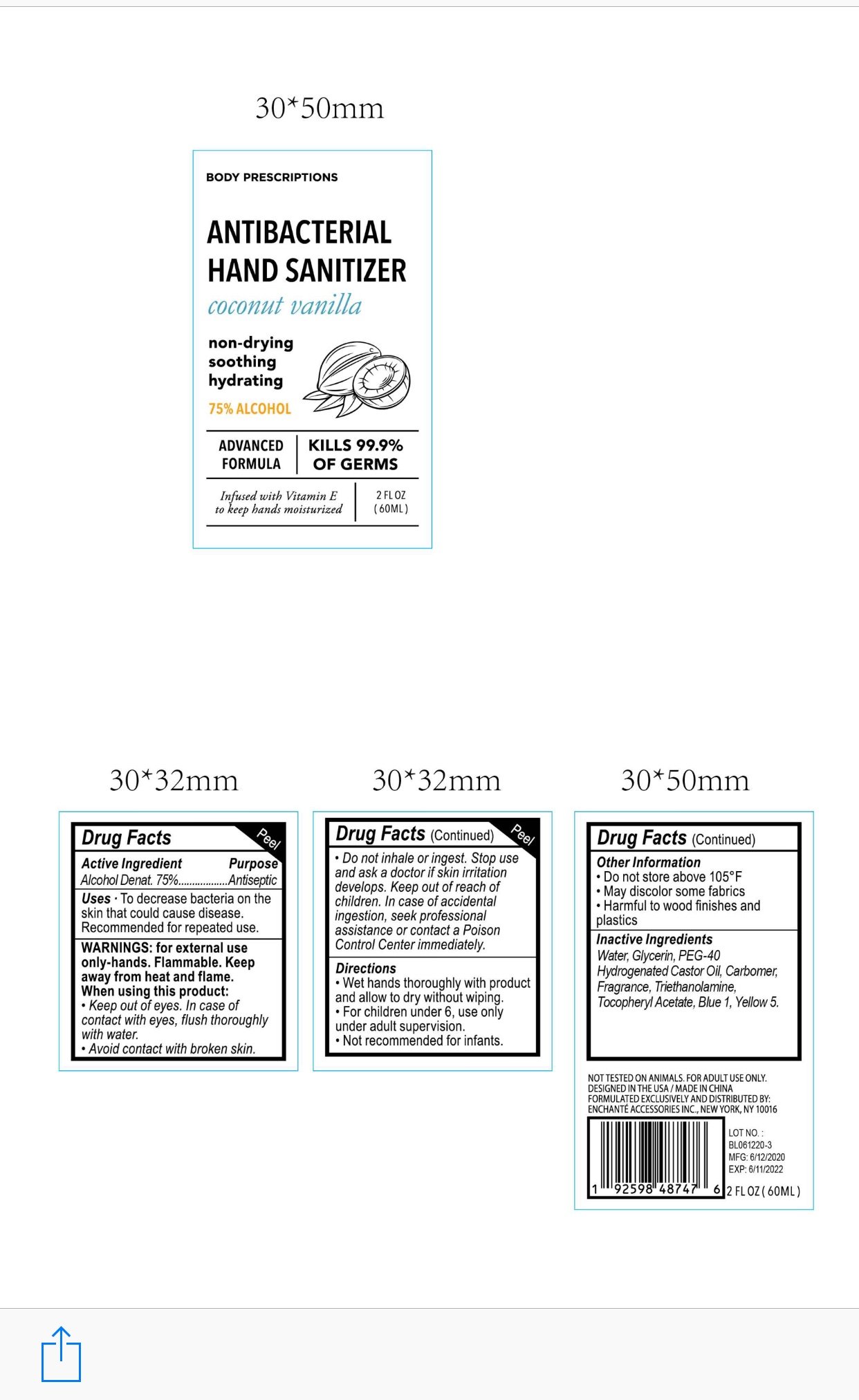
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER COCONUT VANILLA
Alcohol Denat 75%
Antiseptic
To decrease bacteria on the skin that could cause disease
■Recommended for repeated use
For external use only, Hands
Flammable. Keep away from heat and flame
When using this product, keep out of eyes.
In case of contact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest
Stop use and ask a doctor if skin irritation develops
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately
Wet hands thoroughly with product and allow to dry without wiping
For children under 6, use only under adult supervision
Not recommended for infants
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Water, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Tocopheryl Acetate, Yellow 5, Blue 1
| BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER
alcohol kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - ENCHANTE ACCESSORIES INC. (186050696) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Xiamen Chengda Industrial Co.,Ltd | 540042034 | manufacture(50563-210) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.