Puricia
Dosage form: liquid
Ingredients: ALCOHOL 70mL in 100mL
Labeler: PHARMABERG INC.
NDC code: 77302-001
Medically reviewed by Drugs.com. Last updated on May 1, 2024.
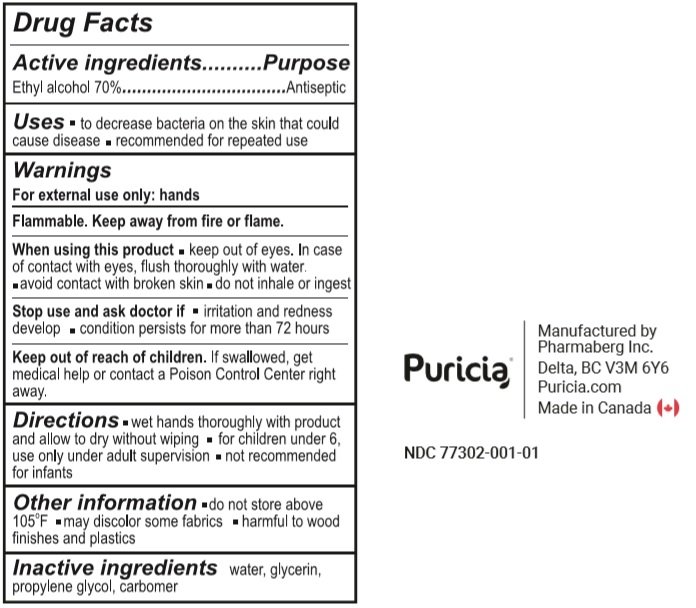
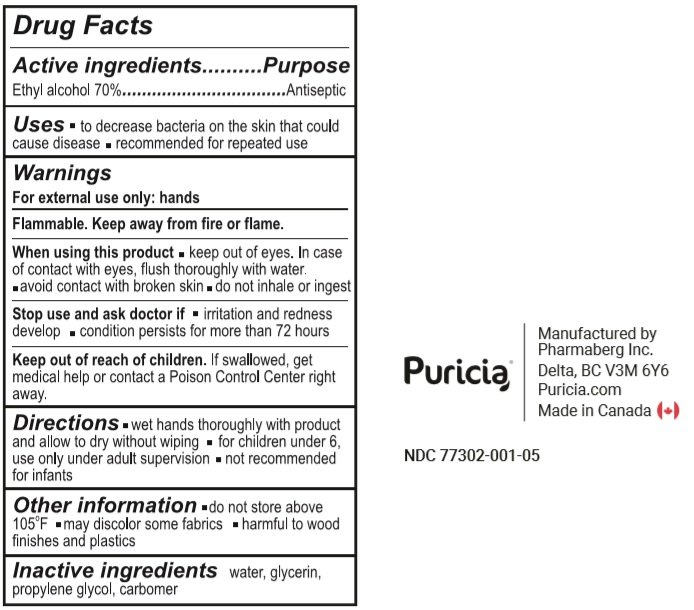
Active Ingredient
Ethyl alcohol 70%
Purpose
Antiseptic
Uses
- To decrease bacteria on the skin that could cause disease.
- Recommended for repeated use.
Warnings
For external use only: hands
Flammable. Keep away from fire or flame.
When using this product
- keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- avoid contact with broken skin.
- do not inhale or ingest.
Stop use and ask a doctor if
- irritation or redness develop.
- condition persists for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Centre right away
Directions
- wet hands thoroughly with product and allow to dry without wiping.
- for children under 6, use only under adult supervision
- not recommended for infants.
Other information
- do not store above 105°F
- may discolor some fabrics.
- harmful to wood finishes and plastics
Inactive ingredients
Water, Glycerin, Propylene Glycol, Carbomer.
| PURICIA
ethyl alcohol 70% liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - PHARMABERG INC. (204355565) |
Revised: 05/2020
Document Id: a460ea98-6015-3253-e053-2a95a90a90ee
Set id: a4a63089-d110-1554-e053-2995a90a0aad
Version: 1
Effective Time: 20200511
PHARMABERG INC.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.