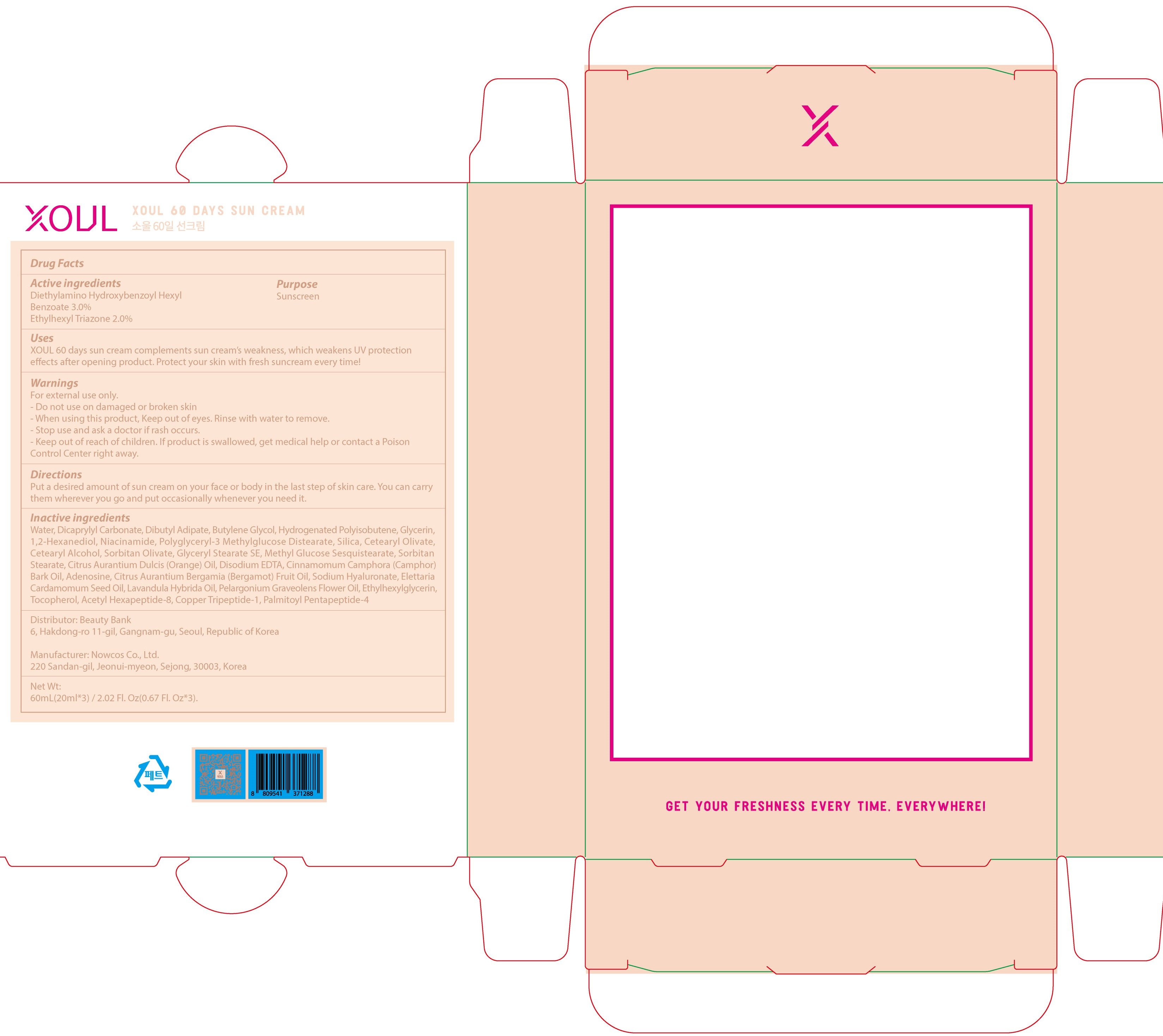
Xoul 60days sun
Dosage form: cream
Ingredients: DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE 0.6g in 20mL, ETHYLHEXYL TRIAZONE 0.4g in 20mL
Labeler: CrossJ
NDC code: 73695-020
Medically reviewed by Drugs.com. Last updated on Mar 6, 2024.
Active ingredients: Diethylamino Hydroxybenzoyl Hexyl Benzoate 3.0%, Ethylhexyl Triazone 2.0%
Inactive ingredients:
Water, Dicaprylyl Carbonate, Dibutyl Adipate, Butylene Glycol, Hydrogenated Polyisobutene, Glycerin, 1,2-Hexanediol, Niacinamide, Polyglyceryl-3 Methylglucose Distearate, Silica, Cetearyl Olivate, Cetearyl Alcohol, Sorbitan Olivate, Glyceryl Stearate SE, Methyl Glucose Sesquistearate, Sorbitan Stearate, Citrus Aurantium Dulcis (Orange) Oil, Disodium EDTA, Cinnamomum Camphora (Camphor) Bark Oil, Adenosine, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Sodium Hyaluronate, Elettaria Cardamomum Seed Oil, Lavandula Hybrida Oil, Pelargonium Graveolens Flower Oil, Ethylhexylglycerin, Tocopherol, Acetyl Hexapeptide-8, Copper Tripeptide-1, Palmitoyl Pentapeptide-4
Purpose: Sunscreen
Warnings:
For external use only.
- Do not use on damaged or broken skin
- When using this product, Keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
KEEP OUT OF REACH OF CHILDREN
Uses:
XOUL 60 days sun cream complements sun cream’s weakness, which weakens UV protection effects after opening product. Protect your skin with fresh suncream every time!
Directions:
Put a desired amount of sun cream on your face or body in the last step of skin care. You can carry them wherever you go and put occasionally whenever you need it.
| XOUL 60DAYS SUN
diethylamino hydroxybenzoyl hexyl benzoate, ethylhexyl triazone cream |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - CrossJ (694918159) |
| Registrant - CrossJ (694918159) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Nowcos.Co.,Ltd | 689914984 | manufacture(73695-020) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.