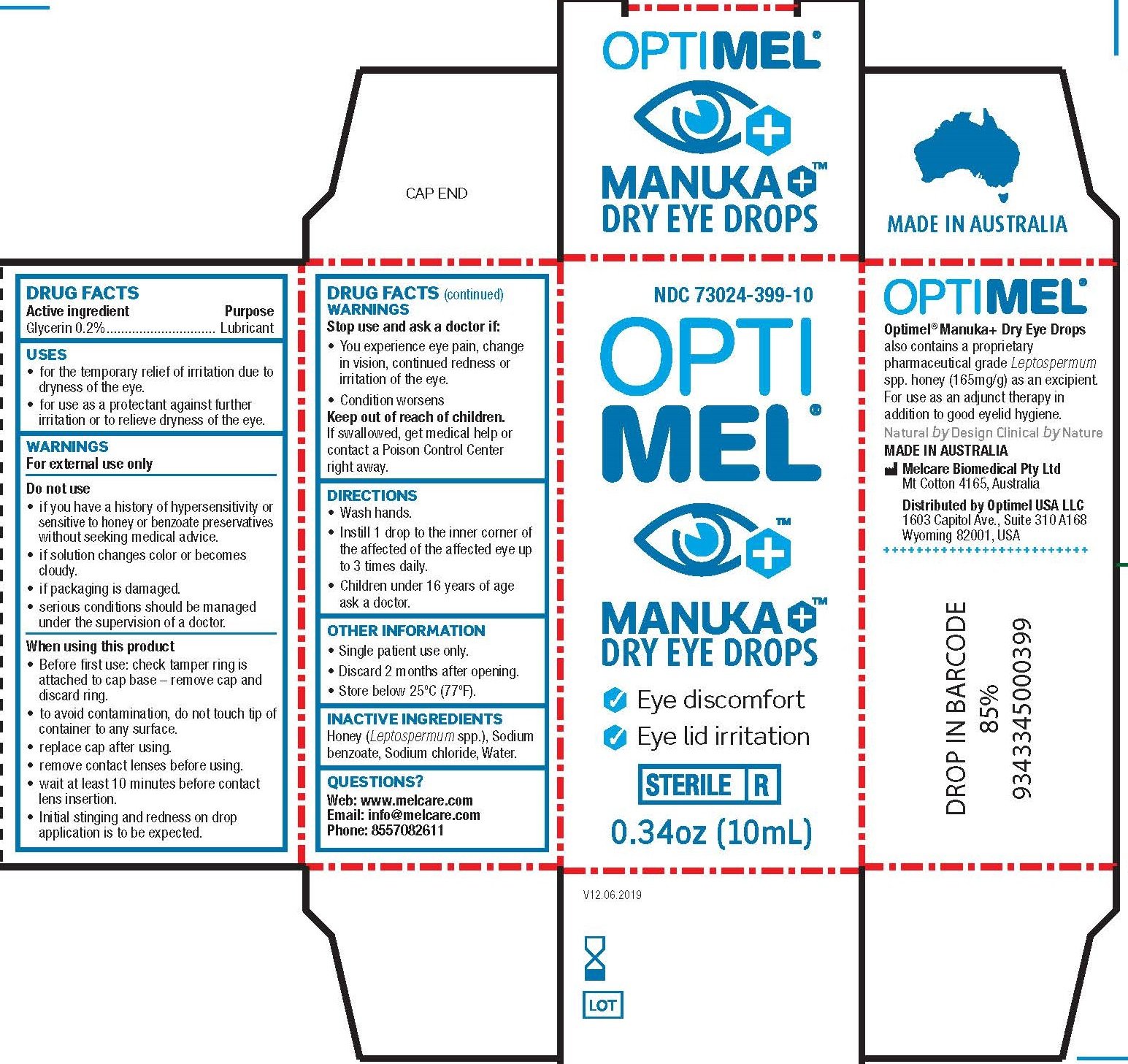
Optimel Manuka Dry Eye Drops
Dosage form: solution/ drops
Ingredients: GLYCERIN 2mg in 1mL
Labeler: Melcare Biomedical Pty Ltd
NDC code: 73024-399
Medically reviewed by Drugs.com. Last updated on Dec 9, 2024.
Drug Facts
Active ingredients
Glycerin 0.2%
Purpose
Lubricant
Uses
- for the temporary relief of burning and irritation due to dryness of the eye.
- for use as a protectant against further irritation or to relieve dryness of the eye.
Warnings
For external use only
Do not use
- if you have a history of hypersensitivity or sensitive to honey or benzoate preservatives without seeking medical advice.
- if solution changes color or becomes cloudy.
- if packaging is damaged.
- serious conditions should be managed under the supervision of a doctor.
When using this product
- Before first use: check tamper ring is attached to cap base – remove cap and discard ring.
- to avoid contamination, do not touch tip of container to any surface.
- replace cap after using.
- remove contact lenses before using.
- wait at least 10 minutes before contact lens insertion.
- Initial stinging and redness on drop application is to be expected.
Stop use and ask a doctor if
- You experience eye pain change in vision, continued redness or irritation of the eye.
- Condition worsens
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wash hands.
- Instill 1 drop to the inner corner of the affected of the affected eye up to 3 times daily.
- Children under 16 years of age ask a doctor.
Other information
- Single patient use only.
- Discard 2 months after opening.
- Store below 25°C (77°F).
Inactive ingredients
Honey ( Leptospermum spp.), Sodium benzoate, Sodium chloride, Water.
Questions?
Web: www.melcare.com
Email: info@melcare.com
Phone: 8557082611
| OPTIMEL MANUKA DRY EYE DROPS
glycerin solution/ drops |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Melcare Biomedical Pty Ltd (743166886) |
Revised: 12/2019
Document Id: 99fd089d-7f62-3903-e053-2a95a90a62f6
Set id: 88c5e2f0-9f7e-62d1-e053-2995a90a9014
Version: 2
Effective Time: 20191218
Melcare Biomedical Pty Ltd
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.