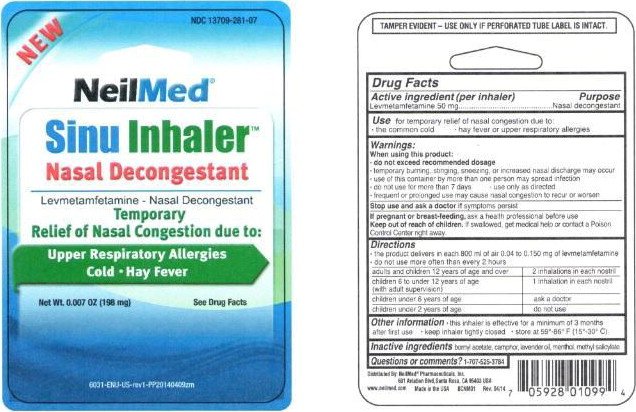
Sinu Inhaler
Dosage form: inhalant
Ingredients: LEVMETAMFETAMINE 50mg
Labeler: NeilMed
NDC code: 13709-281
Medically reviewed by Drugs.com. Last updated on Dec 9, 2024.
Levmetamfetamine 50 mg
Nasal decongestant
- the common cold
- hay fever or upper respiratory allergies
- do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
- do not use for more than 7 days
- Use only as directed
- frequent or prolonged use may cause nasal congestion to recur or worsen
symptoms persist
ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
- the product delivers in each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
| adults and children 12 years of age and over | 2 inhalations in each nostril |
|
children 6 to under 12 years of age (with adult supervision) | 1 inhalation in each nostril |
| children under 6 years of age | ask a doctor |
| children under 2 years of age | do not use |
- this inhaler is effective for a minimum of 3 months after first use
- keep inhaler tightly closed
- store at 59o-86oF (15o- 30oC)
bornyl acetate, camphor, lavender oil, menthol, methyl salicylate
TAMPER EVIDENT - USE ONLY IF PERFORATED TUBE LABEL IS INTACT
DISTRIBUTED BY NeilMed® Pharmaceuticals, Inc.
601 Aviation Blvd., Santa Rosa, CA 95403 USA
www.NeilMed.com Made in the USA BCNM01 Rev. 04/14
NEW
NDC 13709-281-07
NeilMed®
Sinu Inhaler™
Nasal Decongestant
_______________________
Levmetamfetamine - Nasal Decongestant
Temporary
Relief of Nasal Congestion due to:
Upper Respiratory Allergies
Cold • Hay Fever
Net Wt 0.007 OZ (198 mg) See Drug Facts
6031-ENU-US-rev1-PP20140409zm
| SINU INHALER
levmetamfetamine nasal decongestant inhalant |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - NeilMed (783557783) |
| Registrant - Aphena Pharma Solutions-New York, LLC (078573647) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aphena Pharma Solutions-Maryland, LLC | 829739833 | manufacture(13709-281), pack(13709-281), label(13709-281) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.