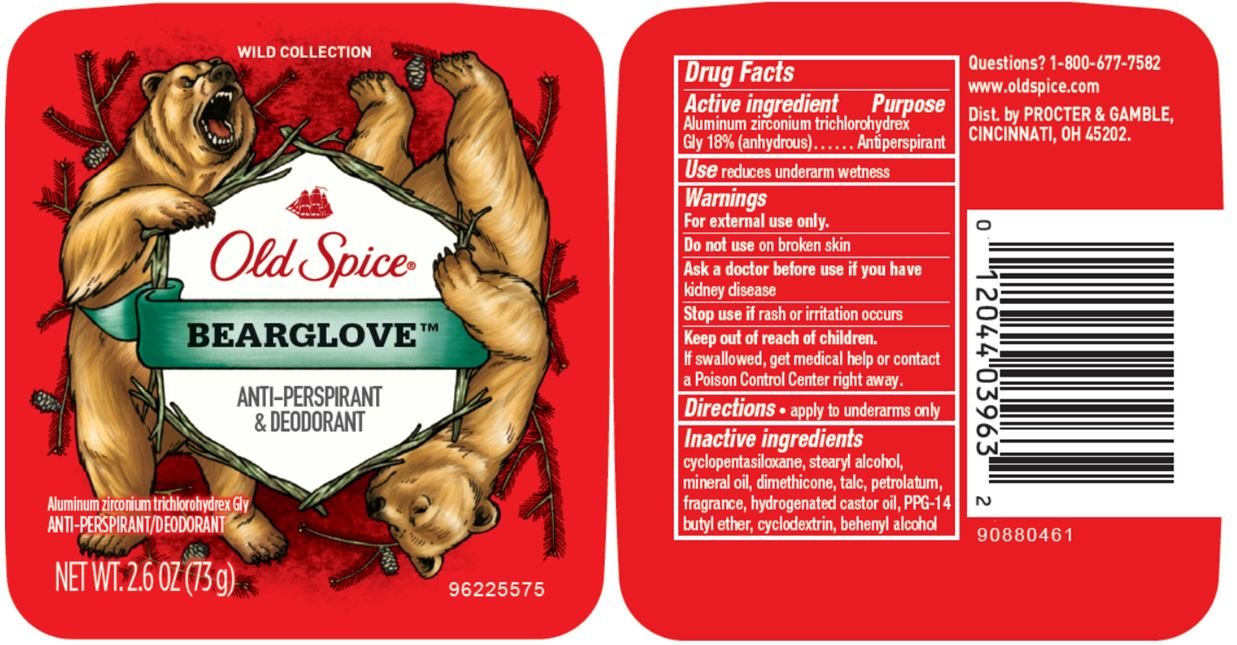
Old Spice Wild Collection Bearglove Invisible
Dosage form: stick
Ingredients: ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY 18g in 100g
Labeler: The Procter & Gamble Manufacturing Company
NDC code: 37000-151
Medically reviewed by Drugs.com. Last updated on Jun 20, 2025.
Bearglove™ Invisible
Drug Facts
Aluminum zirconium trichlorohydrex Gly 18% (anhydrous)
Antiperspirant
reduces underarm wetness
For external use only.
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Stop use if rash or irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- apply to underarms only
cyclopentasiloxane, stearyl alcohol, mineral oil, dimethicone, talc, petrolatum, fragrance, hydrogenated castor oil, PPG-14 butyl ether, cyclodextrin, behenyl alcohol
1-800-677-7582
www.oldspice.com
Dist. by PROCTER & GAMBLE,
CINCINNATI, OH 45202.
| OLD SPICE WILD COLLECTION
BEARGLOVE INVISIBLE
aluminum zirconium trichlorohydrex gly stick |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.