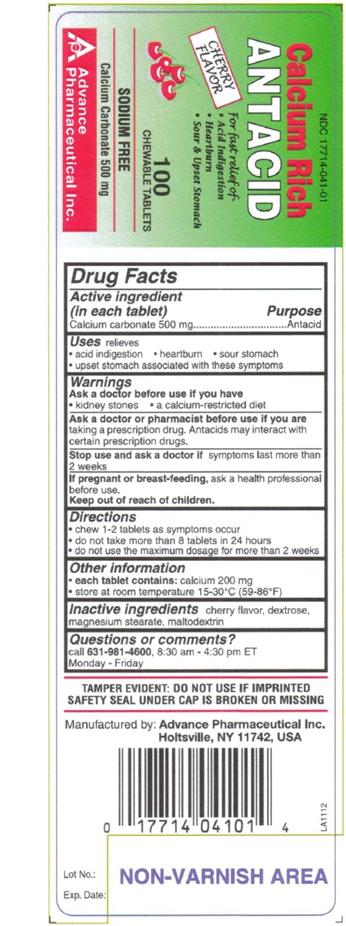
ANTACID
Dosage form: tablet, chewable
Ingredients: CALCIUM CARBONATE 500mg
Labeler: Advance Pharmaceutical Inc.
NDC code: 17714-041
Medically reviewed by Drugs.com. Last updated on Dec 3, 2024.
(in each tablet)
Calcium Carbonate 500 mg
Antacid
relieves
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Ask a doctor before use if you have
- kidney stones
- a calcium-restricted diet
ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than 2 weeks.
If pregnant or breast-feeding, ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
- chew 1-2 tablets as symptoms occurs.
- do not take more than 8 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
- each tablet contains: calcium 200 mg
- store at room temperature 15-30 °C (59-86 °F)
Cherry flavor, dextrose, magnesium stearate, maltodextrin
Call 631-981-4600, Monday-Friday, 8.30 am – 4.30 pm ET
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Manufactured by: Advance Pharmaceutical Inc. Holtsville, NY 11742
| ANTACID
calcium carbonate tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Advance Pharmaceutical Inc. (078301063) |
| Registrant - Advance Pharmaceutical Inc. (078301063) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Advance Pharmaceutical Inc. | 078301063 | manufacture(17714-041) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.