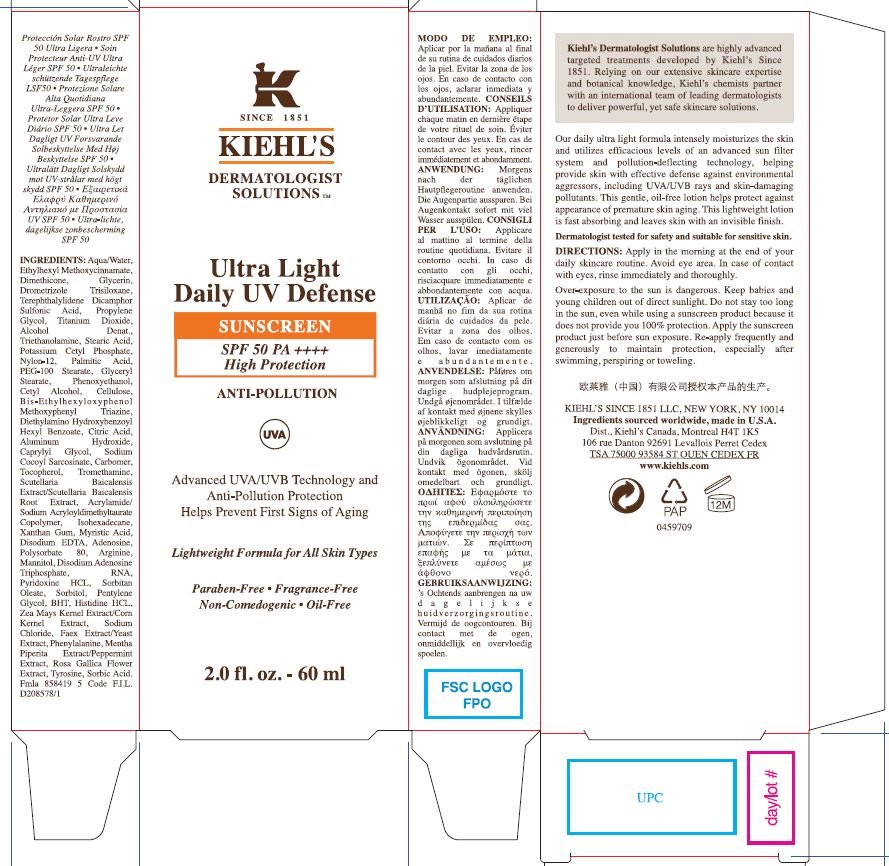
Kiehls Dermatologist Solutions Ultra Light Daily UV Defense Sunscreen SPF 50 High Protection Anti-Pollution Lightweight Formula for All Skin Types
Dosage form: lotion
Ingredients: DROMETRIZOLE TRISILOXANE 40mg in 1mL, TITANIUM DIOXIDE 40mg in 1mL, ECAMSULE 120mg in 1mL, BEMOTRIZINOL 5mg in 1mL, DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE 5mg in 1mL, OCTINOXATE 67.5mg in 1mL
Labeler: L'Oreal USA Products Inc
NDC code: 49967-449
Medically reviewed by Drugs.com. Last updated on Jan 6, 2025.
Drometrizole Trisiloxane
Titanium Dioxide
Terephthalylidene Dicamphor Sulfonic Acid
Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine
Diethylamino Hydroxybenzoyl Hexyl Benzoate
Ethylhexyl Methoxycinnamate
Aqua/Water, Glycerin, Propylene Glycol, Alcohol Denat., Triethanolamine, Stearic Acid, Potassium Cetyl Phosphate, Nylon-12, Palmitic Acid, PEG-100 Stearate, Glyceryl Stearate, Phenoxyethanol, Cetyl Alcohol, Cellulose, Citric Acid, Aluminum Hydroxide, Caprylyl Glycol, Sodium Cocoyl Sarcosinate, Carbomer, Tocopherol, Tromethanime, Scutellaria Baicalensis Extract/Scutellaria Baicalensis Root Extract, Acrylamide/Sodium Acryloyldimethyltaurate Copolymer, Isohexadecane, Xanthan Gum, Myristic Acid, Disodium EDTA, Adenosine, Polysorbate 80, Arginine, Mannitol, Disodium Adenosine Triphosphate, RNA, Pyridoxane HCL, Sorbitan Oleate, Sorbitol, Pentylene Glycol, BHT, Histidine HCL, Zea Mays Kernel Extract/Corn Kernel Extract, Sodium Chloride, Faex Extract/Yeast Extract, Phenylalanine, Mentha Piperita Extract/Perppermint Extract, Rosa Gallica Flower Extract, Tyrosine, Sorbic Acid
Apply in the morning at the end of your daily skincare routine. Avoid eye area. In case of contact with eyes, rinse immediately and thoroughly.
| KIEHLS DERMATOLOGIST SOLUTIONS ULTRA LIGHT DAILY UV DEFENSE SUNSCREEN SPF 50 HIGH PROTECTION ANTI-POLLUTION LIGHTWEIGHT FORMULA FOR ALL SKIN TYPES
drometrizole trisiloxane, titanium dioxide, ecamsule, bemotrizinol, diethylamino hydroxybenzoyl hexyl benzoate and octinoxate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L'Oreal USA Products Inc (002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| L'OREAL USA, INC. | 185931458 | manufacture(49967-449) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.