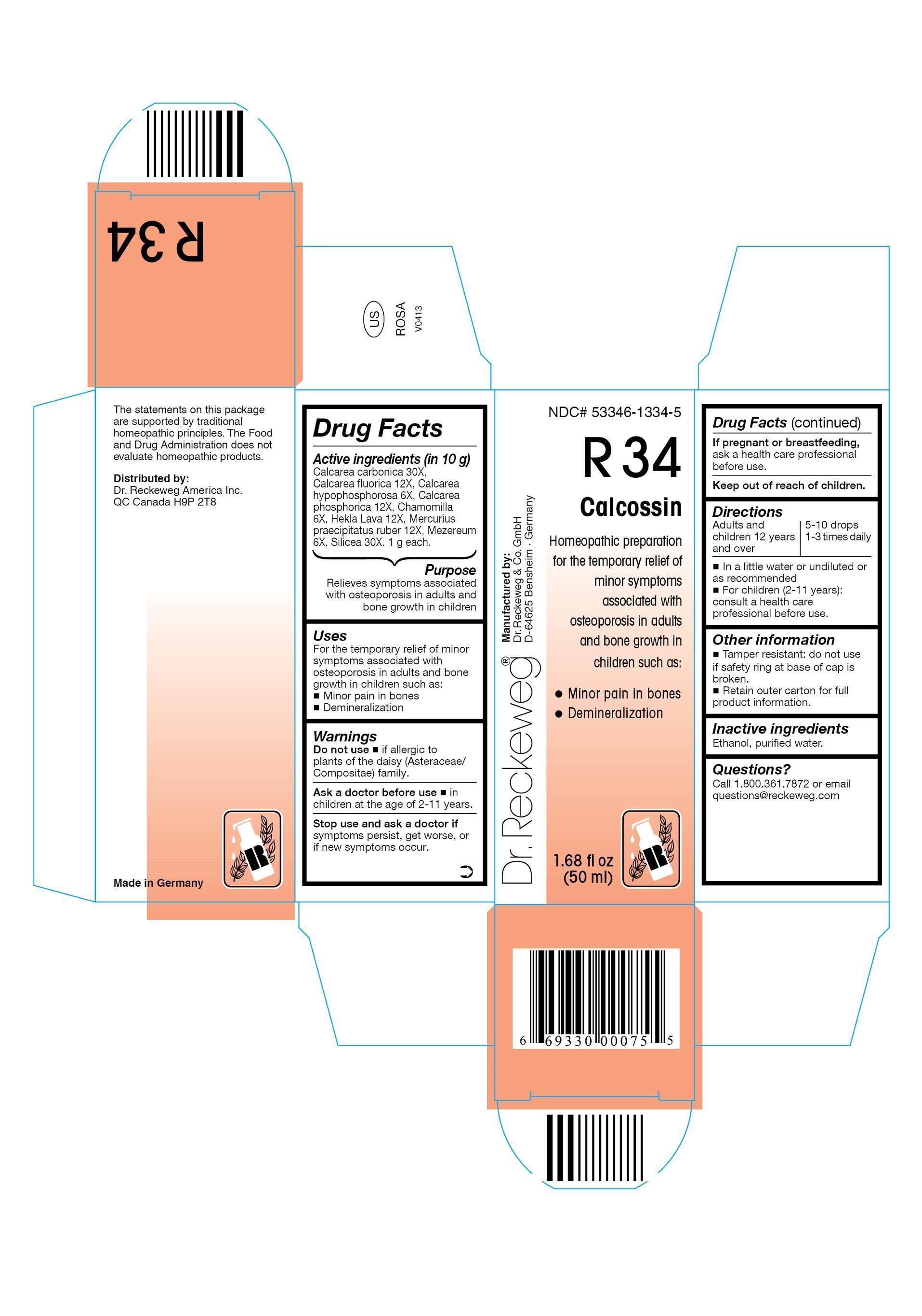
DR. RECKEWEG R34 Calcossin Combination Product
Dosage form: liquid
Ingredients: OYSTER SHELL CALCIUM CARBONATE, CRUDE 30[hp_X] in 50mL, CALCIUM FLUORIDE 12[hp_X] in 50mL, CALCIUM HYPOPHOSPHITE 6[hp_X] in 50mL, CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE 12[hp_X] in 50mL, MATRICARIA RECUTITA 6[hp_X] in 50mL, HEKLA LAVA 12[hp_X] in 50mL, MERCURIC OXIDE 12[hp_X] in 50mL, DAPHNE MEZEREUM BARK 6[hp_X] in 50mL, SILICON DIOXIDE 30[hp_X] in 50mL
Labeler: PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
NDC code: 53346-1334
Active ingredients
Calcarea carbonica 30X, Calcarea fluorica 12X, Calcarea hypophosphorosa 6X, Calcarea phosphorica 12X, Chamomilla 6X, Hekla Lava 12X, Mercurius praecipitatus ruber 12X, Mezereum 6X, Silicea 30X,
1 g each in 10 g.
Purpose
Relieves symptoms associated with osteoporosis in adults and bone growth in children
Uses
For the temporary relief of minor symptoms associated with osteoporosis in adults and bone growth in children such as:
- Minor pain in bones
- Demineralization
Warnings
Do not use
- if allergic to plants of the daisy (Asteraceae/Compositae) family.
Ask a doctor before use
- in children at the age of 2-11 years.
Stop use and ask a doctor if symptoms persist, get worse, or if new symptoms occur.
If pregnant or breastfeeding, ask a health care professional before use.
Keep out of reach of children.
Directions
Adults and children ≥ 12 years 5-10 drops 1-3 times daily in a little water or undiluted or as recommended. For children (2-11 years): consult a health care professional before use.
Other information
- Tamper resistant: do not use if safety ring at base of cap is broken.
- Retain outer carton for full product information.
Inactive ingredients
Ethanol, purified water.
Questions?
Call 1.800.361.7872 or email questions@reckeweg.com
| DR. RECKEWEG R34 CALCOSSIN
COMBINATION PRODUCT
calcarea carbonica 30x, calcarea fluorica 12x, calcarea hypophosphorosa 6x, calcarea phosphorica 12x, chamomilla 6x, hekla lava 12x, mercurius praecipitatus ruber 12x, mezereum 6x, silicea 30x liquid |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO (318602612) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO | 318602612 | manufacture(53346-1334) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.