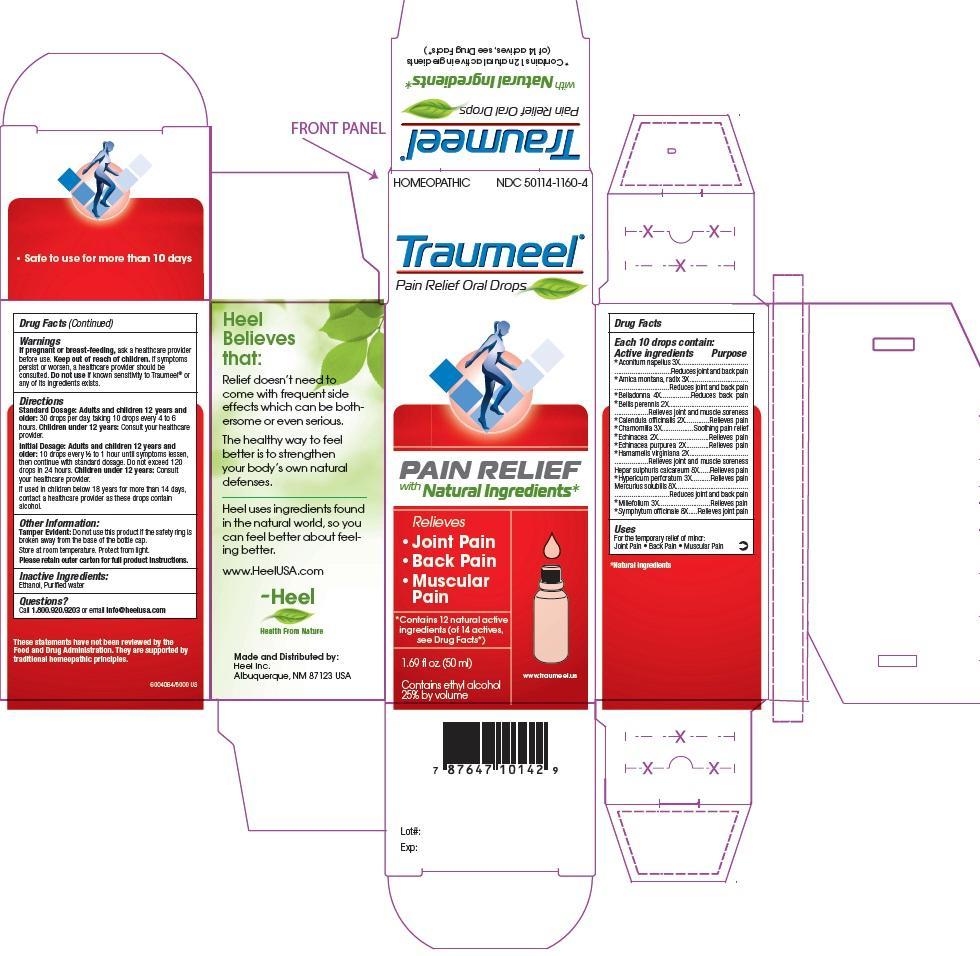
Traumeel
Dosage form: liquid
Ingredients: ACONITUM NAPELLUS 3[hp_X] in 50mL, ARNICA MONTANA ROOT 3[hp_X] in 50mL, ATROPA BELLADONNA 4[hp_X] in 50mL, BELLIS PERENNIS 2[hp_X] in 50mL, CALENDULA OFFICINALIS FLOWERING TOP 2[hp_X] in 50mL, MATRICARIA RECUTITA 3[hp_X] in 50mL, ECHINACEA, UNSPECIFIED 2[hp_X] in 50mL, ECHINACEA PURPUREA 2[hp_X] in 50mL, HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 2[hp_X] in 50mL, CALCIUM SULFIDE 8[hp_X] in 50mL, HYPERICUM PERFORATUM 3[hp_X] in 50mL, MERCURIUS SOLUBILIS 8[hp_X] in 50mL, ACHILLEA MILLEFOLIUM 3[hp_X] in 50mL, COMFREY ROOT 8[hp_X] in 50mL
Labeler: Heel Inc
NDC code: 50114-1160
Keep out of reach of children.
For the temporary relief of minor:
- Joint Pain
- Back Pain
- Muscular Pain
If pregnant or breast-feeding, ask a healthcare provider before use. Keep out of reach of children. If symptoms persist or worsen, a healthcare provider should be consulted. Do not use if known sensitivity to Traumeel® or any of its ingredients exists.
Standard Dosage:
Adults and children 12 years and older: 30 drops per day, taking 10 drops every 4 to 6 hours.
Children under 12 years: Consult your healthcare provider.
Initial Dosage:
Adults and children 12 years and older: 10 drops every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage. Do not exceeed 120 drops in 24 hours.
Children under 12 years: Consult your healthcare provider.
If used in children below 18 years for more than 14 days, contact a healthcare provider as these drops contain alcohol.
Each 10 drops contains as active ingredients: Aconitum napellus 3X, Arnica montana, radix 3X, Belladonna 4X, Bellis perennis 2X, Calendula officinalis 2X, Chamomilla 3X, Echinacea 2X, Echinacea purpurea 2X, Hamamelis virginiana 2X, Hepar sulphuris calcareum 8X, Hypericum perforatum 3X, Mercurius solubilis 8X, Millefolium 3X, Symphytum officinale 8X
Ethanol, Purified Water
Aconitum napellus 3X ..................Reduces joint and back pain
Arnica montana, radix 3X ............Reduces joint and back pain
Belladonna 4X .............................Reduces back pain
Bellis perennis 2X .......................Relieves joint and muscle soreness
Calendula officinalis 2X ...............Relieves pain
Chamomilla 3X ............................Soothing pain relief
Echinacea 2X ..............................Relieves pain
Echinacea purpurea 2X ..............Relieves pain
Hamamelis virginiana 2X ............Relieves joint and muscle soreness
Hepar sulphuris calcareum 8X ....Relieves pain
Hypericum perforatum 3X ...........Relieves pain
Mercurius solubilis 8X .................Reduces joint and back pain
Millefolium 3X .............................Relieves pain
Symphytum officinale 8X ............Relieves joint pain
| TRAUMEEL
aconitum napellus, arnica montana root, atropa belladonna, bellis perennis, calendula officinalis flowering top, matricaria recutita, echinacea, unspecified, echinacea purpurea, hamamelis virginiana root bark/stem bark, calcium sulfide, hypericum perforatum, mercurius solubilis, achillea millefolium and comfrey root liquid |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Heel Inc (102783016) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Heel Inc | 102783016 | manufacture(50114-1160) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.