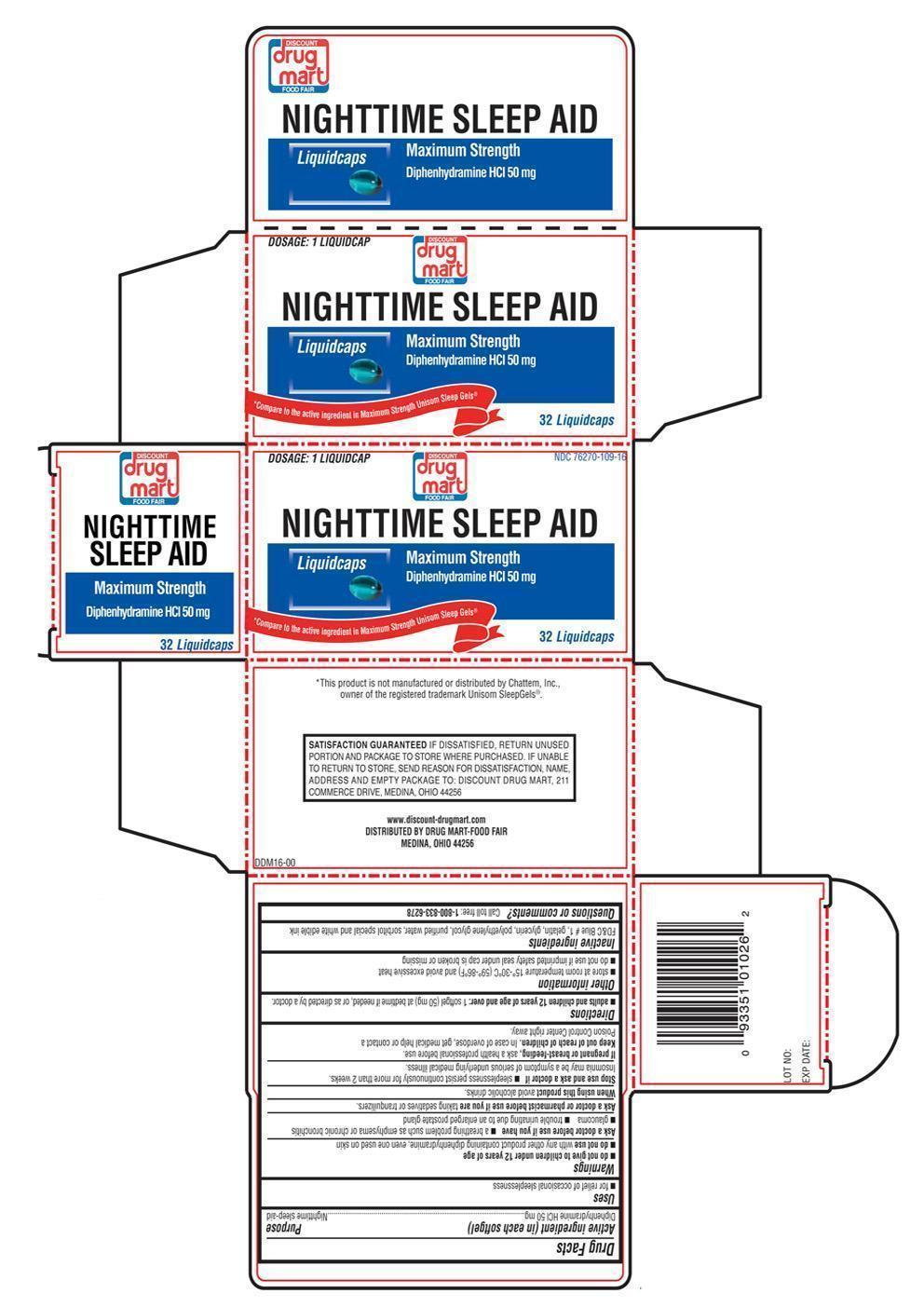
NIGHTTIME SLEEP AID
Dosage form: capsule, liquid filled
Ingredients: DIPHENHYDRAMINE HYDROCHLORIDE 50mg
Labeler: PuraVation Pharmaceuticals Inc
NDC code: 76270-109
Drug Facts
Diphenhydramine HCl 50 mg
Nighttime sleep-aid
- for relief of occasional sleeplessness
- do not give to children under 12 years of age
- do not use with any other product containing diphenhydramine, even one used on skin
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
When using this product avoid alcoholic drinks.
- sleeplessness persist continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- adults and children 12 years of age and over: 1 softgel (50 mg) at bedtime if needed, or as directed by a doctor.
- store at room temperature 15°-30°C (59°-86°F) and avoid excessive heat
- do not use if imprinted safety seal under cap is broken or missing
FD&C blue #1, gelatin, glycerin, polyethylene glycol, purified water, sorbitol special and white edible ink
| NIGHTTIME SLEEP AID
diphenhydramine hydrochloride 50 mg capsule, liquid filled |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - PuraVation Pharmaceuticals Inc (968385034) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Humanwell PuraCap Pharmaceutical (Wuhan), Ltd. | 421293287 | MANUFACTURE(76270-109), ANALYSIS(76270-109) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.