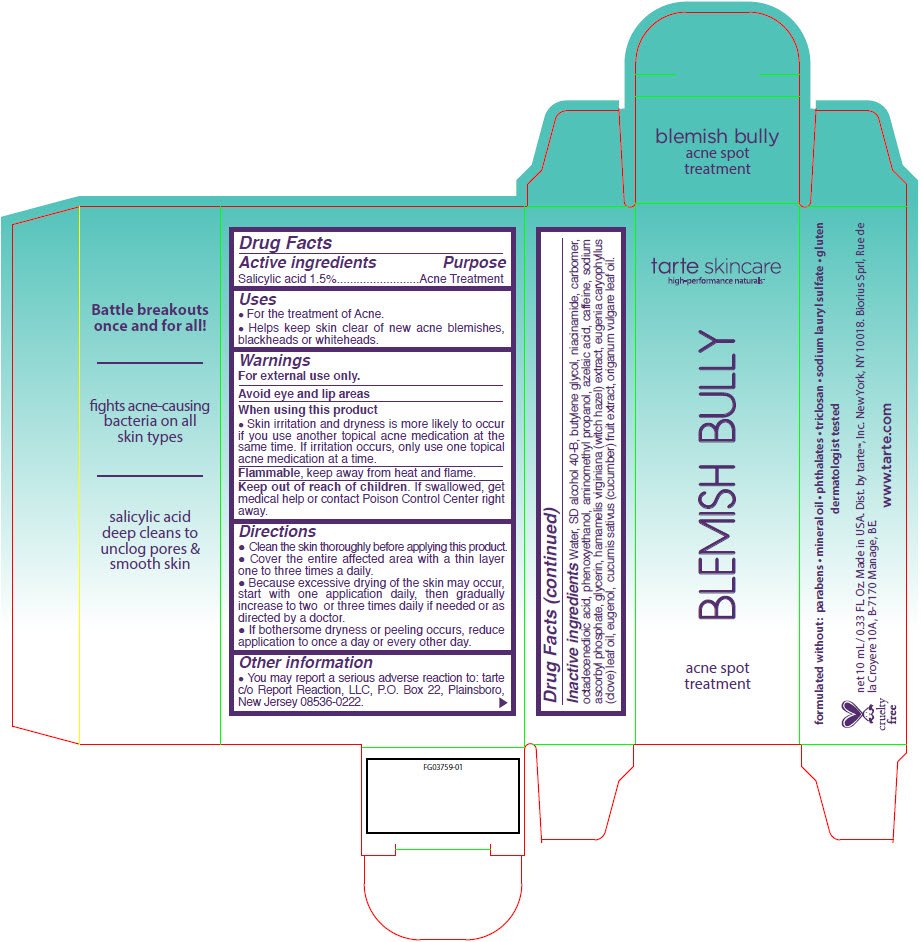
Blemish Bully Acne Spot Treatment
Dosage form: liquid
Ingredients: Salicylic acid 15mg in 1mL
Labeler: Tarte Inc.
NDC code: 51060-125
Medically reviewed by Drugs.com. Last updated on Mar 18, 2024.
Drug Facts
Salicylic acid 1.5%
Acne Treatment
- For the treatment of Acne.
- Helps keep skin clear of new acne blemishes, blackheads or whiteheads.
For external use only.
Avoid eye and lip areas
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Flammable, keep away from heat and flame.
Keep out of reach of children. If swallowed, get medical help or contact Poison Control Center right away.
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times a daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- You may report a serious adverse reaction to: tarte c/o Report Reaction, LLC, P.O. Box 22, Plainsboro, New Jersey 08536-0222.
Water, SD alcohol 40-B, butylene glycol, niacinamide, carbomer, octadecenedioic acid, phenoxyethanol, aminomethyl propanol, azelaic acid, caffeine, sodium ascorbyl phosphate, glycerin, hamamelis virginiana (witch hazel) extract, eugenia caryophyllus (clove) leaf oil, eugenol, cucumis sativus (cucumber) fruit extract, origanum vulgare leaf oil.
Dist. by tarte™, Inc. New York, NY 10018.
| BLEMISH BULLY ACNE SPOT TREATMENT
salicylic acid liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Tarte Inc. (027905186) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.