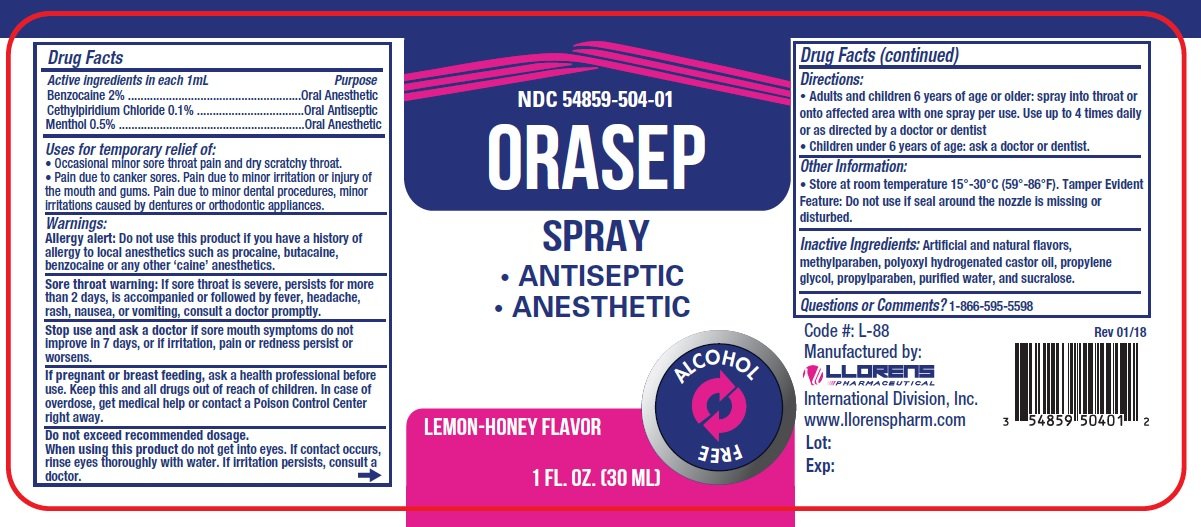
Orasep
Dosage form: liquid
Ingredients: BENZOCAINE 2mg in 100mL, MENTHOL 0.5mg in 100mL, CETYLPYRIDINIUM CHLORIDE 0.1mg in 100mL
Labeler: Llorens Pharmaceutical International Division
NDC code: 54859-504
Medically reviewed by Drugs.com. Last updated on May 6, 2024.
Active Ingredients:
Benzocaine...................... 2% Anesthetic
Cetylpyridinium Chloride..........0.1% Antiseptic
Menthol.................................0.5% Anesthetic
Purpose
Oral anesthetic
Oral antiseptic
Uses for temporary relief of:
- Occasional minor sore throat pain and dry scratch throat.
- Pain due to canker sores. Pain due to minor irritation or injury of the mouth and gums. Pain due to minor dental procedures, minor irritations cause by dentures or orthodontic appliances.
Warnings:
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning: If sore throat is sever, persists for more than2 days, is accompanied or followed by fever, headache, rash, nausea, or vomitings, consult a doctor promptly.
Stop use and ask a doctor if sore mouth symptoms do not improve in 7 days, or if irritation, pain or redness persist or worsens.
Do not exceed recommended dosage.
When using this product do not get into eyes. If contact occurs, rinse eyes thoroughly with water. If irritation persists, consult a doctor.
Keep this and all drugs out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
If pregnant or breast feeding, ask a health professional before use.
Directions:
- Adults and children 6 years of age or older: spray into throat or onto affected area with one spray per use. Use up to 4 times daily or as directed by a doctor or dentist
- Children under 6 years of age: ask a doctor or dentist.
Inactive Ingredients: Artificial and natural flavors, methylparaben, polyoxyl hydrogenated castor oil, propylene glycol, propylparaben, purified water, and sucralose.
Questions or comments? 1-866-595-5598
| ORASEP
benzocaine, menthol, cetylpyridinium chloride liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Llorens Pharmaceutical International Division (037342305) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.