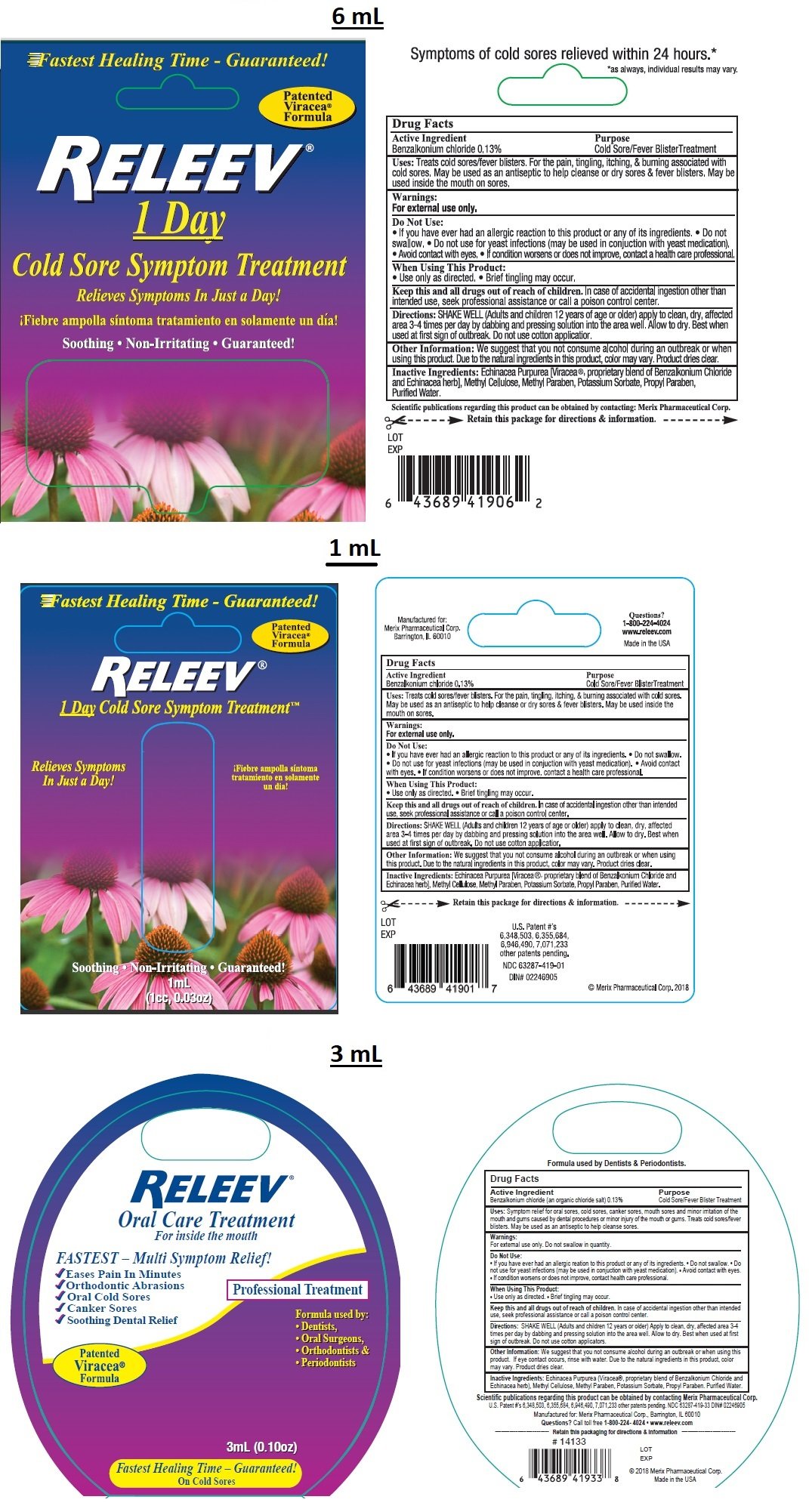
RELEEV Cold Sore Treatment
Dosage form: liquid
Ingredients: BENZALKONIUM CHLORIDE 1.3mg in 1mL
Labeler: Merix Pharmaceutical Corp.
NDC code: 63287-419
Medically reviewed by Drugs.com. Last updated on Dec 23, 2024.
Benzalkonium chloride 0.13%
Cold Sore/ Fever Blister Treatment
Treats cold sores/ fever blisters. For the pain, tingling, itching, & burning associated with cold sores. May be used as an antiseptic to help cleanse or dry cold sores & fever blisters. May be used inside the mouth on sores.
For external use only
Do Not Use:
• If you have ever had an allergic reaction to this product or any of its ingredients. • Do not swallow. • Do not use for yeast infections (may be used in conjunction with yeast medication). • Avoid contact with eyes. • If condition worsens or does not improve, contact a health care professional.
When Using This Product
• Use only as directed • Brief tingling may occur.
Keep this and all drugs out of reach of children. In case of accidental ingestion other than intended use, seek professional assistance or call a poison control center.
SHAKE WELL (Adults and children 12 years of age or older) apply to clean, dry, affected area 3-4 times per day by dabbing and pressing solution into the area well. Allow to dry. Best when used at first sign of outbreak. Do not use cotton applicator.
Echinacea Purpurea [Viracea®, proprietary blend of Benzalkonium Chloride and Echinacea herb], Methyl Cellulose, Methyl Paraben, Potassium Sorbate, Propyl Paraben, Purified Water,
We suggest that you not consume alcohol during an outbreak or when using this product. Due to the natural ingredients in this product, colour may vary. Product dries clear.
| RELEEV COLD SORE TREATMENT
benzalkonium chloride liquid |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Merix Pharmaceutical Corp. (158385687) |
| Registrant - Topical Pharmaceuticals Inc. (831530683) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Topical Pharmaceuticals Inc. | 831530683 | manufacture(63287-419) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.