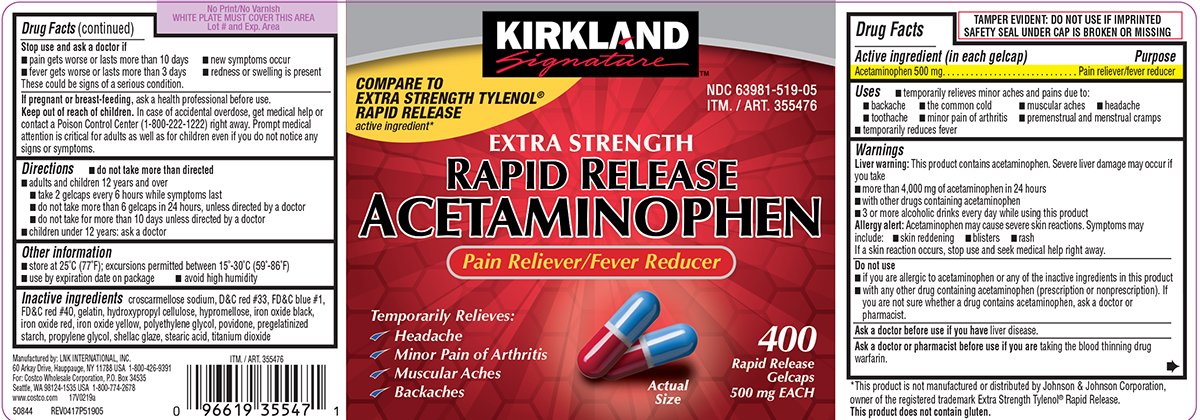
RAPID RELEASE ACETAMINOPHEN Extra Strength
Dosage form: tablet
Ingredients: ACETAMINOPHEN 500mg
Labeler: COSTCO WHOLESALE CORPORATION
NDC code: 63981-519
Medically reviewed by Drugs.com. Last updated on Sep 3, 2024.
Acetaminophen 500 mg
Pain reliever/fever reducer
- temporarily relieves minor aches and pains due to:
- backache
- the common cold
- muscular aches
- headache
- toothache
- minor pain of arthritis
- premenstrual and menstrual cramps
- temporarily reduces fever
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
liver disease.
taking the blood thinning drug warfarin.
- pain gets worse or lasts more than 10 days
- new symptoms occur
- fever gets worse or lasts more than 3 days
- redness or swelling is present
These could be signs of a serious condition.
ask a health professional before use.
In case of accidental overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
do not take more than directed
- adults and children 12 years and over
- take 2 gelcaps every 6 hours while symptoms last
- do not take more than 6 gelcaps in 24 hours, unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: ask a doctor
- store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
- use by expiration date on package
- avoid high humidity
croscarmellose sodium, D&C red #33, FD&C blue #1, FD&C red #40, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, stearic acid, titanium dioxide
KIRKLAND
Signature™
COMPARE TO EXTRA STRENGTH TYLENOL® RAPID RELEASE active ingredient*
NDC 63981-519-05
ITM. / ART. 355476
EXTRA STRENGTH
RAPID RELEASE
ACETAMINOPHEN
Pain Reliever/Fever Reducer
Temporarily Relieves:
✓ Headache
✓Minor Pain of Arthritis
✓Muscular Aches
✓Backaches
Actual
Size
400
Rapid Release
Gelcaps
500 mg EACH
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Extra Strength Tylenol® Rapid Release.
This product does not contain gluten.
Manufactured by: LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788 USA 1-800-426-9391
For: Costco Wholesale Corporation, P.O. Box 34535
Seattle, WA 98124-1535 USA 1-800-774-2678
www.costco.com 17V0219a
50844 REV0417P51905
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING 44-519
| RAPID RELEASE ACETAMINOPHEN
EXTRA STRENGTH
acetaminophen tablet |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - COSTCO WHOLESALE CORPORATION (103391843) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(63981-519) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867837 | MANUFACTURE(63981-519), PACK(63981-519) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(63981-519) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(63981-519) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
