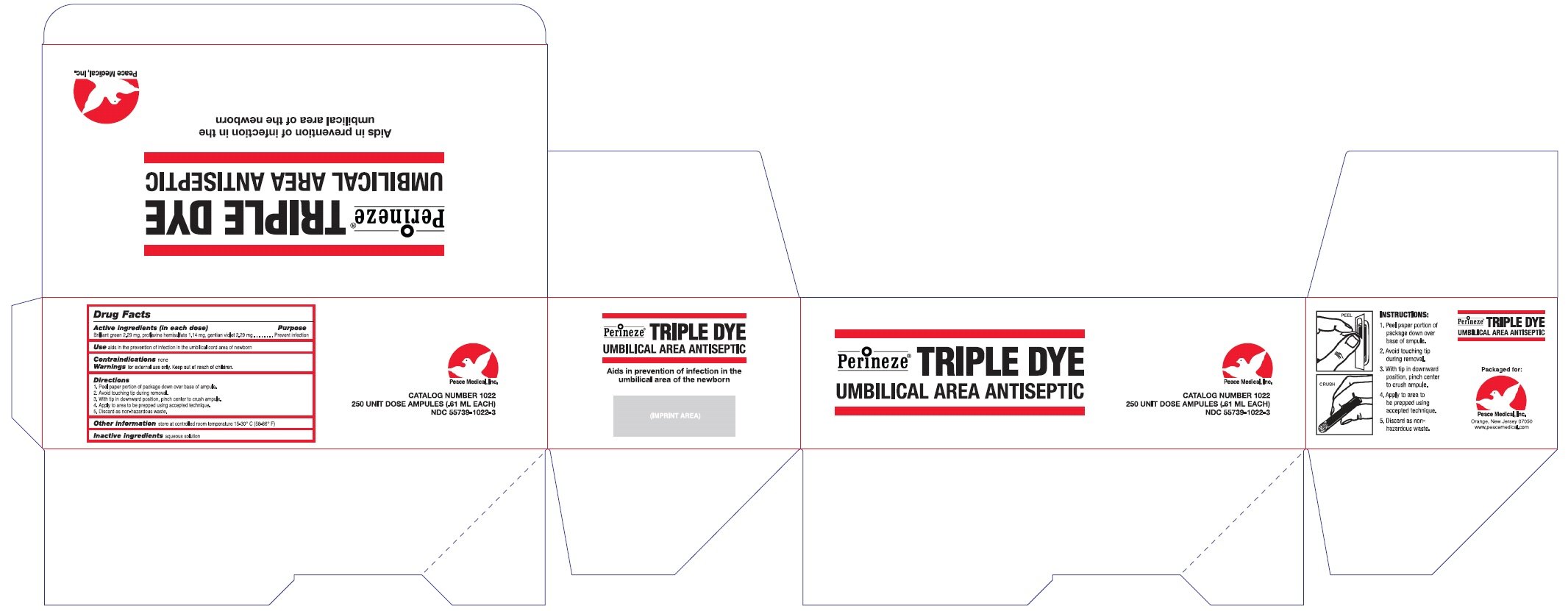
Perineze Triple Dye
Dosage form: solution
Ingredients: BRILLIANT GREEN 2.29mg in 0.61mL, PROFLAVINE HEMISULFATE 1.14mg in 0.61mL, GENTIAN VIOLET 2.29mg in 0.61mL
Labeler: Peace Medical Inc.
NDC code: 55739-1022
Medically reviewed by Drugs.com. Last updated on Sep 18, 2024.
Active Ingredients (in each dose)
Brilliant green 2.29 mg
Proflavine hemisulfate 1.14 mg
gentian violet 2.29 mg
Purpose
Prevent Infection
Use
aids in the prevention of infection in the umbilical cord area of newborn
Contraindications
none
Warnings
for external use only
KEEP OUT OF REACH OF CHILDREN
Directions
- Peel paper portion of package down over base of ampule
- Avoid touching tip during removal
- With tip in downward position, pinch center to crush ampule.
- Apply to area to be prepped using accepted technique.
- Discard as non-hazardous waste.
Other information
store at controlled room temperature 15-30oC (58-86oF)
Inactive ingredients
aqueous solution
| PERINEZE TRIPLE DYE
brilliant green, proflavine hemisulfate, gentian violet solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Peace Medical Inc. (010906881) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Peace Medical Inc. | 010906881 | manufacture | |
Revised: 09/2011
Document Id: 4e7ff19e-48ee-4d46-9b5f-c7ecd303d178
Set id: 074d2603-ec76-49ca-a0ae-79ce8b90d8ab
Version: 1
Effective Time: 20110928
Peace Medical Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.