BreathAway Mouth Rinse Cinnamon
Dosage form: mouthwash
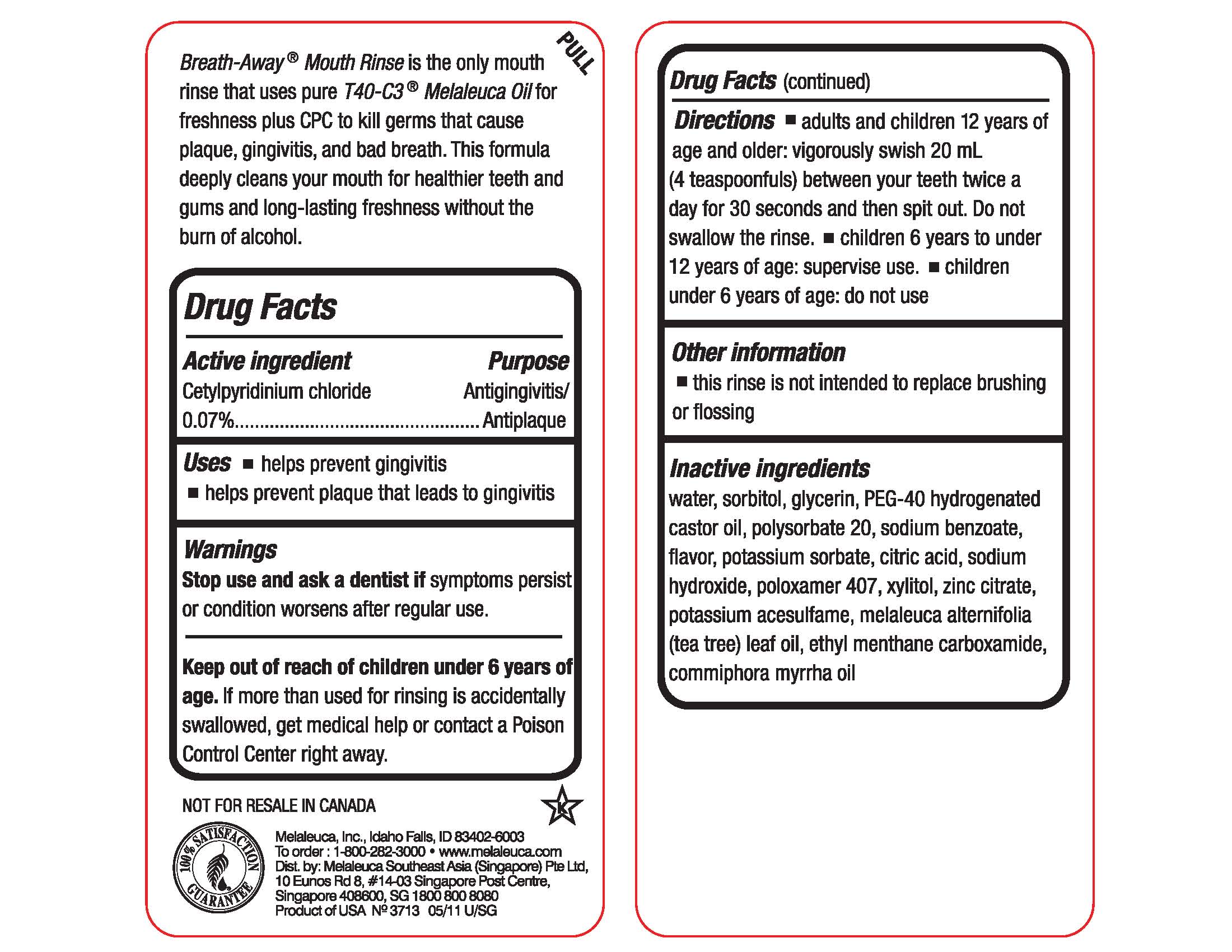
Ingredients: CETYLPYRIDINIUM CHLORIDE 0.3394g in 473mL
Labeler: Melaleuca, Inc.
NDC code: 54473-293
Medically reviewed by Drugs.com. Last updated on Mar 10, 2025.
Active ingredient
Cetylpyridinium chloride 0.07%
Purpose
Antigingivitis/Antiplaque
Use
- helps prevent gingivitis
- helps prevent plaques that leads to gingivitis
Warnings
Stop use and ask a dentist if symptoms persist or condition worsens after regular use.
Keep out of reach of children under 6 years of age. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12years of age and older: vigorously swish 20 mL (4 teaspoonfuls) between your teeth twice a day for 30 seconds and then spit out. Do not swallow the rinse.
- children 6 years to under 12 years of age: supervise use.
- children under 6 years of age: do not use
Inactive ingredients
water, sorbitol, glycerin. PEG-40 hydrogenated castor oil, polysorbate 20, sodium benzoate, flavor, potassium sorbate, citric acid, sodium hydroxide, poloxamer 407, xylitol, zinc citrate, potassium acesulfame, melaleuca alternifolia (tea tree) leaf oil, ethyl menthane carboxamide, commiphora myrrha oil
| BREATHAWAY
MOUTH RINSE CINNAMON
cetylpyridinium chloride mouthwash |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Melaleuca, Inc. (139760102) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Melaleuca, Inc. | 079711683 | manufacture(54473-293) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.