The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Picot Plus Effervescent
Dosage form: powder
Ingredients: sodium bicarbonate 2.485g, citric acid 1.948g, aspirin 500mg
Labeler: Bristol-Myers Squibb de Mexico, S. de R.L. de C.V.
NDC code: 64613-5600
Drug FactsDrug Facts
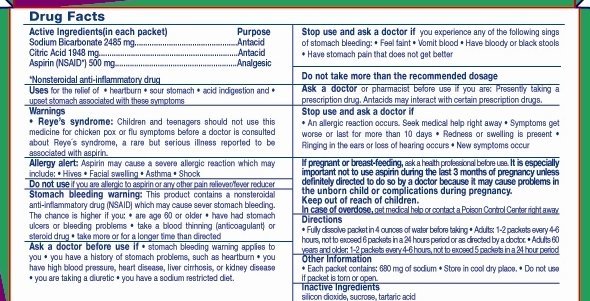
Active Ingredients (in each packet) Purpose
Sodium Bicarbonate 2.485 g ..........................Antacid
Citric Acid 1.948 g ........................................Antacid
Aspirin (NSAID*) 500 mg ..............................Analgesic
*Nonsterioidal anti-inflammatory drug
Uses for the relief of * heartburn * sour stomach * acid
indigestion * headache * upset stomach and for temporary
relief of minor aches and pains
Warnings
Reye's syndrome: Children and teenagers should not use
this medicine for chicken pox or flu symptoms before a doctor
is consulted about Reye's syndrome, a rare but serious
illness reported to be associated with aspirin.
Allergy alert: Aspirin may cause a severe allergic reaction
which may include * Hives * Facial swelling * Asthma * Shock
Do not use if you are allergic to aspirin or any other pain
reliever/fever reducer
Stomach bleeding warning: This product contains a nonsteroidal anti-
inflammatory drug (NSAID) which may cause severe stomach bleeding.
The chance is higher if you are age 60 or older * have had
Drug Facts (continued)
stomach ulcers or bleeding problems * take a blood thinner (anti-
coagulant) or steroid drug * have 3 or more alcoholic drinks every
day while using this product * take more or for a longer time than directed
Ask a doctor before use if *stomach bleeding warning applies to
you * you have a history of stomach problems, such as heartburn *
you have high blood pressure, heart disease, liver cirrhosis, or kidney
disease * you are taking a diuretic * you have a sodium restricted diet.
Stop use and ask a doctor if you experience any of the following
signs of stomach bleeding * Feel faint * Vomit blood * Have bloody
or black stools * Have stomach pain that does not get better
Do not take more than the recommended dosage
Ask a doctor or pharmacist before use if you are: Presently taking a
prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if
An allergic reaction occurs. Seek medical help right away *
Symptoms get worse or last for more than 10 days *
Redness or swelling is present * Ringing in the ears or loss
of hearing occurs * New symptoms occur
If pregnant or breast-feeding, ask a health professional
before use. It is especially important not to use aspirin
during the last 3 months of pregnancy unless definitely
Drug Facts (continued)
directed to do so by a doctor because it may cause problems
in the unborn child or complications during pregnancy.
Keep out of the reach of children.
In case of overdose, get medical help or contact a Poison
Control Center right away
Directions
*Fully dissolve packet in 4 ounces of water before taking *
Adults: 1 - 2 packets every 4 - 6 hours, not to exceed 6 packets
in a 24 hour period or as directed by a doctor. * Adults 60
years and older: 1 - 2 packets every 4 - 6 hours, not to exceed 5
packets in a 24 hour period
Other Information
*Each packet contains 680 mg of sodium * Store in cool dry
place.
Inactive Ingredients
silicon dioxide, sucrose, tartaric acid
Active Ingredients (in each packet) Purpose
Sodium Bicarbonate 2.485 g ..........................Antacid
Citric Acid 1.948 g ........................................Antacid
Aspirin (NSAID*) 500 mg ..............................Analgesic
*Nonsterioidal anti-inflammatory drug
Uses for the relief of * heartburn * sour stomach * acid
indigestion * headache * upset stomach and for temporary
relief of minor aches and pains
Warnings
Reye's syndrome: Children and teenagers should not use
this medicine for chicken pox or flu symptoms before a doctor
is consulted about Reye's syndrome, a rare but serious
illness reported to be associated with aspirin.
Allergy alert: Aspirin may cause a severe allergic reaction
which may include * Hives * Facial swelling * Asthma * Shock
Do not use if you are allergic to aspirin or any other pain
reliever/fever reducer
Stomach bleeding warning: This product contains a nonsteroidal anti-
inflammatory drug (NSAID) which may cause severe stomach bleeding.
The chance is higher if you are age 60 or older * have had
Drug Facts (continued)
stomach ulcers or bleeding problems * take a blood thinner (anti-
coagulant) or steroid drug * have 3 or more alcoholic drinks every
day while using this product * take more or for a longer time than directed
Ask a doctor before use if *stomach bleeding warning applies to
you * you have a history of stomach problems, such as heartburn *
you have high blood pressure, heart disease, liver cirrhosis, or kidney
disease * you are taking a diuretic * you have a sodium restricted diet.
Stop use and ask a doctor if you experience any of the following
signs of stomach bleeding * Feel faint * Vomit blood * Have bloody
or black stools * Have stomach pain that does not get better
Do not take more than the recommended dosage
Ask a doctor or pharmacist before use if you are: Presently taking a
prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if
An allergic reaction occurs. Seek medical help right away *
Symptoms get worse or last for more than 10 days *
Redness or swelling is present * Ringing in the ears or loss
of hearing occurs * New symptoms occur
If pregnant or breast-feeding, ask a health professional
before use. It is especially important not to use aspirin
during the last 3 months of pregnancy unless definitely
Drug Facts (continued)
directed to do so by a doctor because it may cause problems
in the unborn child or complications during pregnancy.
Keep out of the reach of children.
In case of overdose, get medical help or contact a Poison
Control Center right away
Directions
*Fully dissolve packet in 4 ounces of water before taking *
Adults: 1 - 2 packets every 4 - 6 hours, not to exceed 6 packets
in a 24 hour period or as directed by a doctor. * Adults 60
years and older: 1 - 2 packets every 4 - 6 hours, not to exceed 5
packets in a 24 hour period
Other Information
*Each packet contains 680 mg of sodium * Store in cool dry
place.
Inactive Ingredients
silicon dioxide, sucrose, tartaric acid
| PICOT PLUS
EFFERVESCENT
antacid and pain reliever powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bristol-Myers Squibb de Mexico, S. de R.L. de C.V. (823646211) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bristol-Myers Squibb de Mexico, S. de R.L. de C.V | 823646211 | manufacture | |
Revised: 05/2010
Document Id: 7c9552a7-f15b-42ab-a545-106aa51af1a4
Set id: 80ee1531-0c55-47ab-a39d-0cbdec2796fa
Version: 4
Effective Time: 20100528
Bristol-Myers Squibb de Mexico, S. de R.L. de C.V.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.