Novocain: Package Insert / Prescribing Info
Package insert / product label
Generic name: procaine hydrochloride
Dosage form: injection, USP
Drug class: Local injectable anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Novocain Description
NOVOCAIN, procaine hydrochloride, is benzoic acid, 4-amino-, 2-(diethylamino) ethyl ester, monohydrochloride, the ester of diethylaminoethanol and aminobenzoic acid, with the following structural formula:
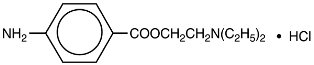
It is a white crystalline, odorless powder that is freely soluble in water, but less soluble in alcohol. Each mL contains 100 mg procaine hydrochloride and 4 mg acetone sodium bisulfite as antioxidant. DO NOT USE SOLUTIONS IF CRYSTALS, CLOUDINESS, OR DISCOLORATION IS OBSERVED. EXAMINE SOLUTIONS CAREFULLY BEFORE USE. REAUTOCLAVING INCREASES LIKELIHOOD OF CRYSTAL FORMATION.
Novocain - Clinical Pharmacology
NOVOCAIN stabilizes the neuronal membrane and prevents the initiation and transmission of nerve impulses, thereby effecting local anesthesia. NOVOCAIN lacks surface anesthetic activity. The onset of action is rapid (2 to 5 minutes) and the duration of action is relatively short (average 1 to 1½ hours), depending upon the anesthetic technique, the type of block, the concentration, and the individual patient.
NOVOCAIN is readily absorbed following parenteral administration and is rapidly hydrolyzed by plasma cholinesterase to aminobenzoic acid and diethylaminoethanol.
A vasoconstrictor may be added to the solution of NOVOCAIN to promote local hemostasis, delay systemic absorption, and increase duration of anesthesia.
Contraindications
Spinal anesthesia with NOVOCAIN is contraindicated in patients with generalized septicemia: sepsis at the proposed injection site; certain diseases of the cerebrospinal system, e.g., meningitis, syphilis; and a known hypersensitivity to the drug, drugs of a similar chemical configuration, or aminobenzoic acid or its derivatives.
The decision as to whether or not spinal anesthesia should be used in an individual case should be made by the physician after weighing the advantages with the risks and possible complications.
Warnings
RESUSCITATIVE EQUIPMENT AND DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHENEVER ANY LOCAL ANESTHETIC DRUG IS USED. Spinal anesthesia should only be administered by those qualified to do so.
Large doses of local anesthetics should not be used in patients with heartblock.
Reactions resulting in fatality have occurred on rare occasions with the use of local anesthetics, even in the absence of a history of hypersensitivity.
Usage in Pregnancy. Safe use of NOVOCAIN has not been established with respect to adverse effects on fetal development. Careful consideration should be given to this fact before administering this drug to women of childbearing potential particularly during early pregnancy. This does not exclude the use of the drug at term for obstetrical analgesia. Vasopressor agents (administered for the treatment of hypotension or added to the anesthetic solution for vasoconstriction) should be used with extreme caution in the presence of oxytocic drugs as they may produce severe, persistent hypertension with possible rupture of a cerebral blood vessel.
Solutions which contain a vasoconstrictor should be used with extreme caution in patients receiving drugs known to produce alterations in blood pressure (i.e., monoamine oxidase inhibitors (MAOI), tricyclic antidepressants, phenothiazines, etc.), as either severe sustained hypertension or hypotension may occur.
Local anesthetic procedures should be used with caution when there is inflammation and/or sepsis in the region of the proposed injection.
Contains acetone sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Precautions
Standard textbooks should be consulted for specific techniques and precautions for various spinal anesthetic procedures.
The safety and effectiveness of a spinal anesthetic depend upon proper dosage, correct technique, adequate precautions, and readiness for emergencies. The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and possible adverse effects. Tolerance varies with the status of the patient. Debilitated, elderly patients, or acutely ill patients should be given reduced doses commensurate with their weight and physical status. Reduced dosages are also indicated for obstetric delivery and patients with increased intra-abdominal pressure.
The decision whether or not to use spinal anesthesia in the following disease states depends on the physician’s appraisal of the advantages as opposed to the risk: cardiovascular disease (i.e., shock, hypertension, anemia, etc.), pulmonary disease, renal impairment, metabolic or endocrine disorders, gastrointestinal disorders (i.e., intestinal obstruction, peritonitis, etc.), or complicated obstetrical deliveries.
NOVOCAIN SHOULD BE USED WITH CAUTION IN PATIENTS WITH KNOWN DRUG ALLERGIES AND SENSITIVITIES. A thorough history of the patient’s prior experience with NOVOCAIN or other local anesthetics as well as concomitant or recent drug use should be taken (see CONTRAINDICATIONS). NOVOCAIN should not be used in any condition in which a sulfonamide drug is being employed since aminobenzoic acid inhibits the action of sulfonamides.
Solutions containing a vasopressor should be used with caution in the presence of diseases which may adversely affect the cardiovascular system.
NOVOCAIN should be used with caution in patients with severe disturbances of cardiac rhythm, shock or heartblock.
Adverse Reactions/Side Effects
Systemic adverse reactions involving the central nervous system and the cardiovascular system usually result from high plasma levels due to excessive dosage, rapid absorption, or inadvertent intravascular injection. In addition, use of inappropriate doses or techniques may result in extensive spinal blockade leading to hypotension and respiratory arrest.
A small number of reactions may result from hypersensitivity, idiosyncrasy, or diminished tolerance to normal dosage.
Excitatory CNS effects (nervousness, dizziness, blurred vision, tremors) commonly represent the initial signs of local anesthetic systemic toxicity. However, these reactions may be very brief or absent in some patients in which case the first manifestation of toxicity may be drowsiness or convulsions merging into unconsciousness and respiratory arrest.
Cardiovascular system reactions include depression of the myocardium, hypotension (or sometimes hypertension), bradycardia, and even cardiac arrest.
Allergic reactions are characterized by cutaneous lesions of delayed onset, or urticaria, edema, and other manifestations of allergy. The detection of sensitivity by skin testing is of limited value. As with other local anesthetics, hypersensitivity, idiosyncrasy and anaphylactoid reactions have occurred rarely. The reaction may be abrupt and severe and is not usually dose related.
The following adverse reactions may occur with spinal anesthesia: Central Nervous System: postspinal headache, meningismus, arachnoiditis, palsies, or spinal nerve paralysis. Cardiovascular: hypotension due to vasomotor paralysis and pooling of the blood in the venous bed. Respiratory: respiratory impairment or paralysis due to the level of anesthesia extending to the upper thoracic and cervical segments. Gastrointestinal: nausea and vomiting.
Treatment of Reactions. Toxic effects of local anesthetics require symptomatic treatment: there is no specific cure. The physician should be prepared to maintain an airway and to support ventilation with oxygen and assisted or controlled respiration as required. Supportive treatment of the cardiovascular system includes intravenous fluids and, when appropriate, vasopressors (preferably those that stimulate the myocardium, such as ephedrine). Convulsions may be controlled with oxygen and by the intravenous administration of diazepam or ultrashort-acting barbiturates or a short-acting muscle relaxant (succinylcholine). Intravenous anticonvulsant agents and muscle relaxants should only be administered by those familiar with their use and only when ventilation and oxygenation are assured. In spinal and epidural anesthesia, sympathetic blockade also occurs as a pharmacological reaction, resulting in peripheral vasodilation and often hypotension. The extent of the hypotension will usually depend on the number of dermatomes blocked. The blood pressure should therefore be monitored in the early phases of anesthesia. If hypotension occurs, it is readily controlled by vasoconstrictors administered either by the intramuscular or the intravenous route, the dosage of which would depend on the severity of the hypotension and the response to treatment.
Novocain Dosage and Administration
As with all local anesthetics, the dose of NOVOCAIN varies and depends upon the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered. For specific techniques and procedures, refer to standard textbooks.
| Extent of Anesthesia | NOVOCAIN 10% Solution | Total Dose (mg) | Site of Injection (lumbar interspace) |
|
| Volume of 10% Solution (mL) | Volume of Dilution (mL) |
|||
| Perineum | 0.5 | 0.5 | 50 | 4th |
| Perineum and lower extremities | 1 | 1 | 100 | 3rd or 4th |
| Up to costal margin | 2 | 1 | 200 | 2nd, 3rd or 4th |
The diluent may be sterile normal saline, sterile distilled water, spinal fluid; and for hyperbaric technique, sterile dextrose solution.
The usual rate of injection is 1 mL per 5 seconds. Full anesthesia and fixation usually occur in 5 minutes.
STERILIZATION
The drug in intact ampuls is sterile. The preferred method of destroying bacteria on the exterior of ampuls before opening is heat sterilization (autoclaving). Immersion in antiseptic solution is not recommended.
Autoclave at 15-pound pressure, at 121°C (250°F), for 15 minutes. The diluent dextrose may show some brown discoloration due to caramelization.
Protect solutions from light.
How is Novocain supplied
|
List |
Container |
Concentration |
Fill |
Quantity |
|
1810 |
Uni-Amp® Unit Dose Pak |
10% |
2 mL |
25 |
| 1810 | Single-Dose Ampuls | 10% | 2 mL | 25 |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Revised: July, 2005
|
©Hospira 2005 |
EN-1078 |
Printed in USA |
Hospira, Inc., Lake Forest, IL 60045 USA
| Novocain
procaine hydrochloride injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hospira, Inc. |
More about Novocain (procaine)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- Drug class: local injectable anesthetics
- Breastfeeding
