Tacrolimus FDA Alerts
The FDA Alerts below may be specifically about tacrolimus or relate to a group or class of drugs which include tacrolimus.
MedWatch Safety Alerts are distributed by the FDA and published by Drugs.com. Following is a list of possible medication recalls, market withdrawals, alerts and warnings.
Recent FDA Alerts for tacrolimus
Astellas Issues Recall of One Lot of Prograf 0.5 mg and One Lot of Astagraf XL 0.5 mg Because Bottles Shipped to U.S. May Contain Empty Capsules
NORTHBROOK, IL, Dec. 23, 2024 – Astellas Pharma US, Inc. (Head of US Commercial: Michael Petroutsas, "Astellas") is voluntarily recalling one lot of Prograf® 0.5mg (tacrolimus) and one lot of Astagraf XL® 0.5mg (tacrolimus extended-release) capsules to the consumer level. These products are being recalled because bottles may contain empty capsules.
Risk Statement
Transplant patients who consume empty Prograf or Astagraf XL capsules may experience initiation of rejection of the transplanted organ, tissue, or cells, due to underimmunosuppression. In the case of life sustaining organ transplants such as a heart transplant (for which there is no permanent substitute such as hemodialysis in the case of a failed kidney transplant) if the transplant fails, the consequences of rejection initiated by ingesting empty capsules may be fatal. To date, Astellas has not received any reports of adverse events related to this recall.
Prograf and Astagraf XL are immunosuppressive medicines, used in conjunction with other medicines, to help prevent organ transplant rejection. Prograf is used in people who have had kidney, heart, liver, or lung transplants and Astagraf XL is indicated for use in people with kidney transplants.
The affected lot numbers and expiration dates are:
|
PRODUCT DESCRIPTION |
NDC |
LOT NO. |
EXP. DATE |
|
Prograf® (tacrolimus) 0.5 mg capsules
100 capsules per bottle |
0469-0607-73 |
0E3353D |
03/2026 |
|
PRODUCT DESCRIPTION |
NDC |
LOT NO. |
EXP. DATE |
|
Astagraf XL® (tacrolimus extended-release capsules) 0.5 mg capsules
30 capsules per bottle |
0469-0647-73 |
0R3092A |
03/2026 |
No other formulations or doses of the product are impacted, and sufficient supply of unaffected stock is available to replace the recalled lots. Product was distributed nationwide to wholesale and retail outlets.
Astellas is notifying its customers via a drug recall notification letter and is arranging for the return of impacted product. Wholesalers or pharmacists with questions about the recall process should contact 1-877-575-3437 during office hours 9 am to 5 pm (EST), Monday through Friday.
Patients that have an affected lot should contact their physician or healthcare provider. Patients and physicians with questions should contact Astellas Medical Information at 1-800-727-7003 During office hours from 9 am to 5:30 pm EST, Monday through Friday.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Astellas
Astellas is a global life sciences company committed to turning innovative science into VALUE for patients. We provide transformative therapies in disease areas that include oncology, ophthalmology, urology, immunology and women's health. Through our research and development programs, we are pioneering new healthcare solutions for diseases with high unmet medical need. Learn more at www.astellas.com.
Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management’s current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas’ intellectual property rights by third parties. Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
FDA is Changing the Therapeutic Equivalence Rating for Accord Healthcare Inc.’s Generics of Prograf (tacrolimus) Oral Capsules
September 18, 2023 -- Based on new data, the U.S. Food and Drug Administration is changing the therapeutic equivalence rating for tacrolimus oral capsule products manufactured by Accord Healthcare Inc. under abbreviated new drug application 091195. These drugs are indicated for the prevention of organ rejection in adult patients receiving kidney, liver, or heart transplants, and in pediatric patients receiving liver transplants.
FDA is concerned that the peak blood concentration of tacrolimus for Accord Healthcare Inc.’s generic tacrolimus oral capsules may be increased compared to the brand-name drug, Prograf (tacrolimus), creating a risk of toxicity. However, according to the new data, there were no significant differences in the trough blood levels, indicating no increased risk for organ rejection.
In 2011, FDA determined that tacrolimus products qualified as narrow therapeutic index drugs, and in 2012, updated recommendations for the design of bioequivalence studies to support therapeutic equivalence for tacrolimus generics. This change led to concerns from the transplant community regarding the substitutability of FDA-approved generic tacrolimus oral capsules that were approved prior to 2012, for both their therapeutic equivalence to the brand-name drug, Prograf (tacrolimus) and other approved generics. FDA funded a number of studies to investigate these concerns. Multiple post-approval studies signaled a potential issue with the bioequivalence of Accord Healthcare Inc.’s tacrolimus oral capsules and this led FDA to fund an additional study by BioPharma Services USA. FDA reviewed the results of the bioequivalence study by BioPharma along with other evidence. These studies indicate that Accord Healthcare Inc.’s tacrolimus oral capsules may deliver drug to the body at a higher maximum concentration compared to Prograf’s tacrolimus oral capsules. The increased peak concentration may result in patients having too much tacrolimus in their body compared to Prograf, which may result in signs or symptoms of tacrolimus toxicity (see Figures 1–3).
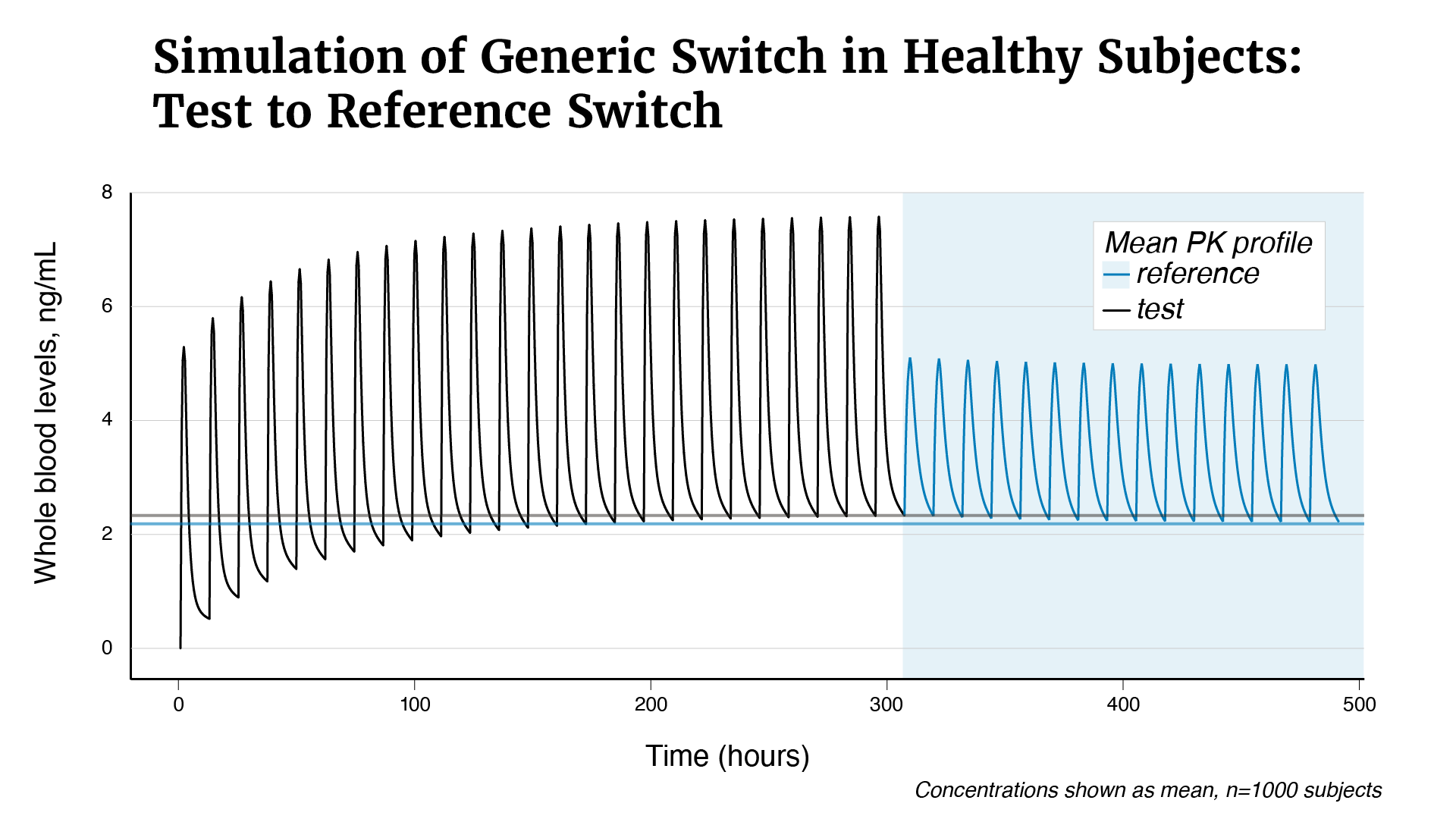
Figure 1. Based on the tacrolimus whole blood levels after a single dose oral administration of Accord (test) and Prograf (reference) tacrolimus oral capsules, a simulation was conducted to demonstrate the impact of switching a subject stabilized on Accord (as determined by the achievement of steady state with multiple subsequent doses of Accord every 12 hours) to Prograf.
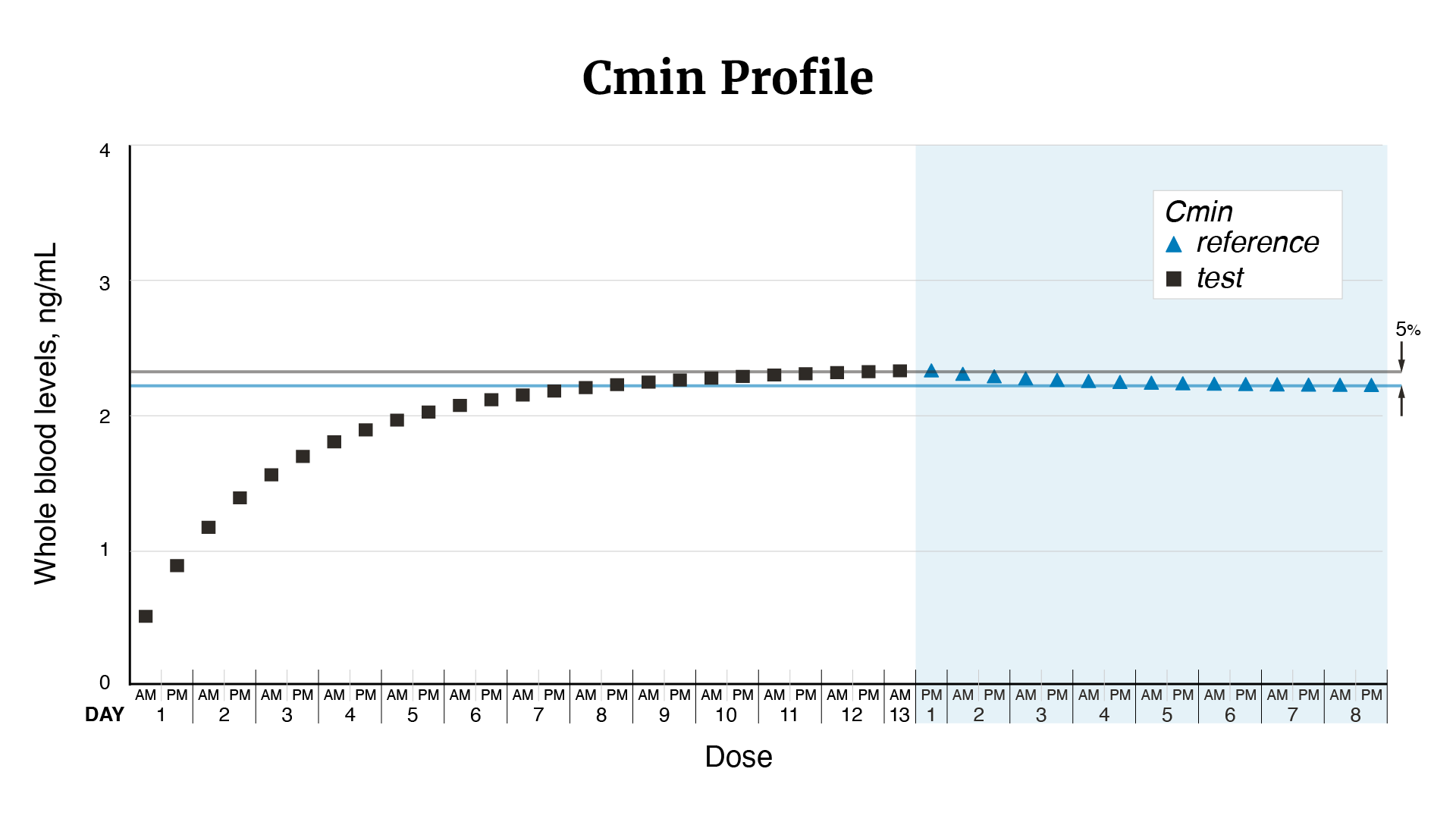
Figure 2. From Figure 1, the minimum tacrolimus whole blood levels (or Cmin or the trough concentration) after each dose of Accord (test) and Prograf (reference) tacrolimus oral capsules (i.e., the concentration at 12 hours when the subsequent dose is given). Horizontal lines represent the steady state Cmin value for each treatment.
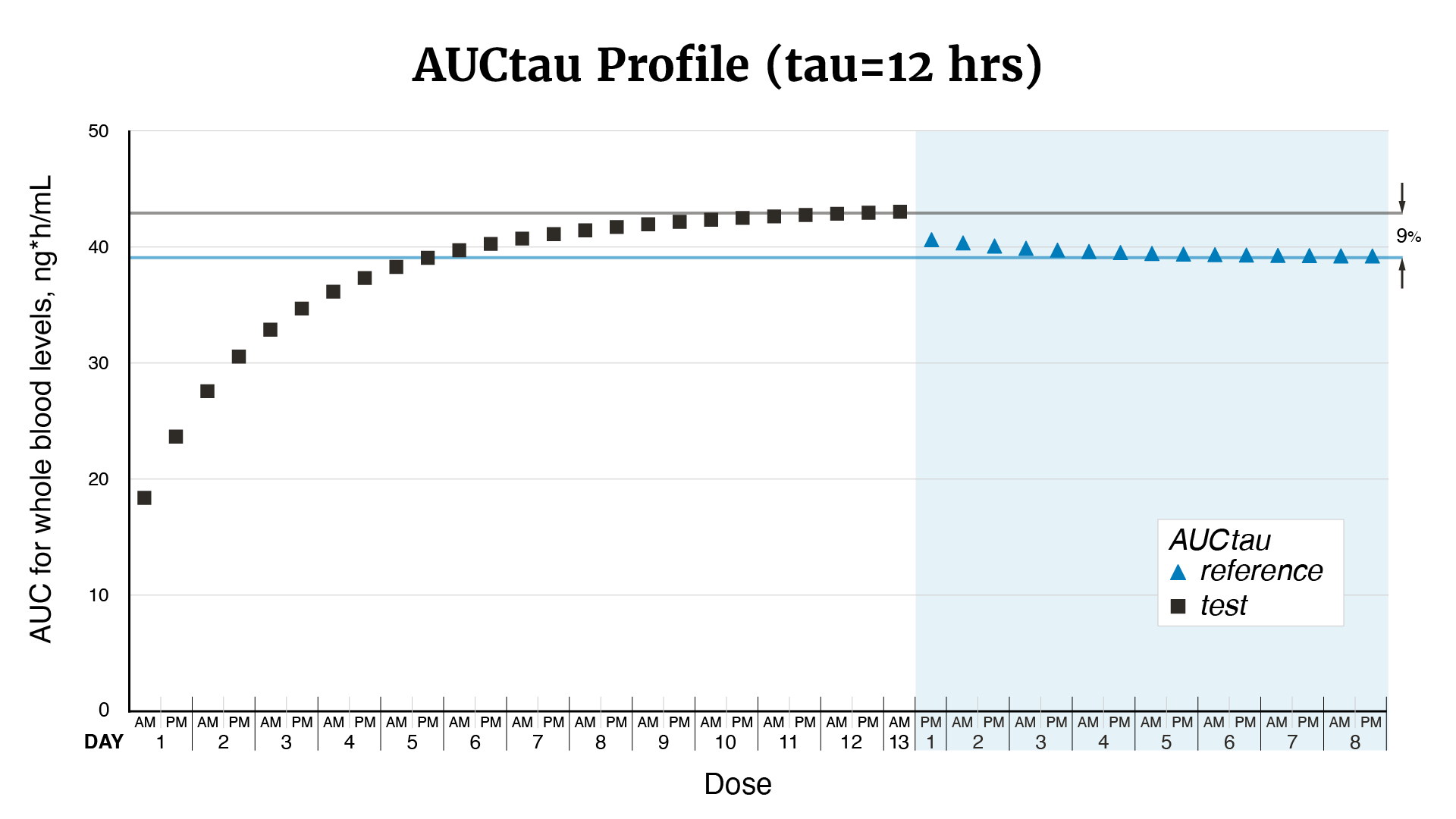
Figure 3. From Figure 1, the overall area-under-the-curve associated with each dose of Accord (test) and Prograf (reference) tacrolimus oral capsules (or AUCtau where tau is 12 hours). Horizontal lines represent the steady state AUCtau value for each treatment.
Potential harms to patients from higher concentrations of tacrolimus include kidney damage (nephrotoxicity); high blood pressure (hypertension); nerve damage (neurotoxicity), such as seizures, tremors, or headache; and elevated potassium levels (hyperkalemia). However, FDA is not aware of post-marketing issues regarding safety or efficacy for Accord Healthcare Inc.’s tacrolimus oral capsules.
As a result of its review of this new information, FDA has changed the therapeutic equivalence rating for Accord Healthcare Inc.’s tacrolimus oral capsules from AB to BX. A BX rating means that the data are insufficient to show that Accord Healthcare Inc.’s tacrolimus oral capsules provide the same therapeutic effect as Prograf (tacrolimus) oral capsules. This means that Accord Healthcare Inc.’s tacrolimus oral capsules are still FDA-approved and can be prescribed, but are no longer recommended as automatically substitutable at the pharmacy (or by a pharmacist) for Prograf (tacrolimus) oral capsules.
Prograf (tacrolimus) oral capsules and generic tacrolimus oral capsules manufactured by companies other than Accord Healthcare Inc. are not affected by this issue. Other tacrolimus dosage forms are also not affected by this issue, including tacrolimus extended-release oral capsules, tacrolimus granules for oral suspension, tacrolimus injection products for intravenous use, and tacrolimus topical ointments.
Patients can determine the manufacturer of their tacrolimus oral capsules by contacting their pharmacy. Patients currently taking Accord Healthcare Inc.’s tacrolimus oral capsules should not make changes to their treatment, except in consultation with their health care professional. Patients should also inform their health care professional if they have experienced any problems that may be related to taking Accord Healthcare Inc.’s tacrolimus oral capsules.
FDA encourages health care professionals and consumers to report adverse events or quality problems with these or any medications to FDA’s MedWatch Adverse Event Reporting program:
-
Complete and submit the report online; or
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Related Information
- Questions and Answers Regarding Tacrolimus Oral Capsules (generic of Prograf) Made by Accord Healthcare Inc.
- FDA Response to Citizen Petition from Belcher Pharmaceuticals, LLC (FDA-2020-P-1247)
Source: FDA
Tacrolimus Active Pharmaceutical Ingredient [API]
[Posted 05/18/2006] Spectrum Laboratory Products and FDA notified healthcare professionals of the recall of the active pharmaceutical ingredient tacrolimus, an immunosuppressive drug used to prevent rejections of transplanted solid organs such as heart or kidney, after learning that some lots of the ingredient are subpotent. Spectrum's tacrolimus API has been used by pharmacies for compounding purposes. The use of sub-potent tacrolimus in compounded drugs for transplant recipients may lead to sub-therapeutic tacrolimus blood levels and an unacceptable increased risk of solid organ transplant rejection. Patients receiving tacrolimus for solid organ transplant should not stop taking their medication, but rather should check with their physician or pharmacist. This recall does not apply to tacrolimus marketed in finished dosage form as Prograf (Astellas Pharma, US) or to Prograf oral capsules that have been used for compounding.[May 11, 2006 - Press Release - Spectrum Laboratory Products]
More tacrolimus resources
- Tacrolimus oral and injection Consumer Information
- Tacrolimus (Intravenous) Advanced Consumer Information
- Tacrolimus (Oral) Advanced Consumer Information
- Tacrolimus AHFS DI Monograph
- Tacrolimus Extended-Release Tablets Consumer Information
- Tacrolimus Capsules Consumer Information
- Tacrolimus Extended-Release Capsules Consumer Information
- Tacrolimus Granules for Suspension Consumer Information
- Tacrolimus Injection Consumer Information
- Tacrolimus Prescribing Information
- Tacrolimus ER Capsules Prescribing Information
