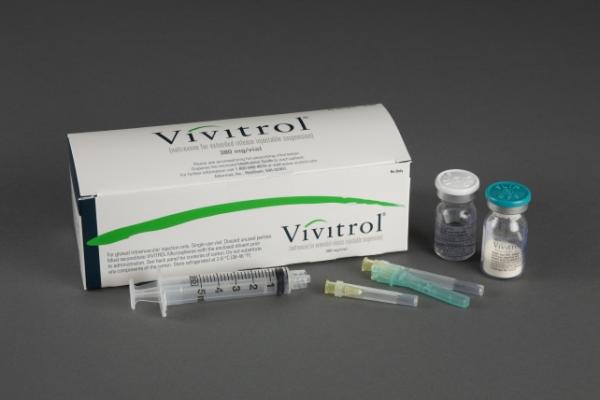
Vivitrol Dosage
Generic name: naltrexone 380mg in 4mL;
Dosage form: injection
Drug class: Drugs used in alcohol dependence
Medically reviewed by Drugs.com. Last updated on May 22, 2024.
Important Dosage and Administration Information
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial.
Prior to initiating VIVITROL, an opioid-free duration of a minimum of 7–10 days is recommended for patients, to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization.
The recommended dose of VIVITROL is 380 mg delivered intramuscularly (deep) as a gluteal injection every 4 weeks or once a month, alternating buttocks for each subsequent injection, using the carton components provided. The needles provided in the carton are customized needles. VIVITROL must not be injected using any other needle. The needle lengths (either 1 1/2 or 2 inches) may not be adequate in every patient because of body habitus. Body habitus should be assessed prior to each injection for each patient to assure that needle length is adequate for intramuscular administration. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. Healthcare providers should ensure that the VIVITROL injection is given correctly, and should consider alternate treatment for those patients whose body habitus precludes an intramuscular gluteal injection with one of the provided needles.
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver, at the initial VIVITROL injection and with each subsequent injection. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose after VIVITROL treatment is discontinued, at the end of the VIVITROL dosing interval (i.e., near the end of the month that VIVITROL was administered), or after a dose of VIVITROL is missed, strongly consider prescribing naloxone for the emergency treatment of opioid overdose.
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Reinitiation of Treatment in Patients Previously Discontinued
There are no data to specifically address reinitiation of treatment. Patients reinitiating treatment with VIVITROL should be opioid-free at the time of dose administration.
Switching From Oral Naltrexone
There are no systematically collected data that specifically address the switch from oral naltrexone to VIVITROL.
Switching from Buprenorphine, Buprenorphine/Naloxone, or Methadone
There are no systematically collected data that specifically address the switch from buprenorphine or methadone to VIVITROL; however, review of postmarketing case reports have indicated that some patients may experience severe manifestations of precipitated withdrawal when being switched from opioid agonist therapy to opioid antagonist therapy. Patients transitioning from buprenorphine or methadone may be vulnerable to precipitation of withdrawal symptoms for as long as 2 weeks. Healthcare providers should be prepared to manage withdrawal symptomatically with non-opioid medications.
Directions for Use
VIVITROL must be prepared and administered by a healthcare provider.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
To ensure proper dosing, it is important that you follow the preparation and administration instructions outlined in this document.
VIVITROL must be suspended only in the diluent supplied in the carton and must be administered only with one of the administration needles supplied in the carton. The microspheres, diluent, preparation needle, and an administration needle with needle protection device are required for preparation and administration. Two thin-walled 1 1/2-inch needles with needle protection device and two 2-inch thin-walled needles with needle protection device have been provided to accommodate varying patient body habitus. For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass. For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle contacting the periosteum. Either needle may be used for patients with average body habitus. A spare administration needle of each size is provided in case of clogging. Do not substitute any other components for the components of the carton.
Prior to preparation, allow drug to reach room temperature (approximately 45 minutes).
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial.
Keep out of reach of children.
Prepare and administer the VIVITROL suspension using aseptic technique.
WARNING: To reduce the risk of a needlestick:
- Do not intentionally disengage the needle protection device.
- Discard bent or damaged needle into a sharps container and use the spare needle provided. Do not attempt to straighten the needle or engage needle protection device if the needle is bent or damaged.
- Do not mishandle the needle protection device in a way that could lead to protrusion of the needle.
- Do not use free hand to press sheath over needle.
THE CARTON SHOULD NOT BE EXPOSED TO TEMPERATURES EXCEEDING 25 °C (77 °F).
The entire carton should be stored in the refrigerator (2 °C to 8 °C, 36 °F to 46 °F). Unrefrigerated, VIVITROL microspheres can be stored at temperatures not exceeding 25 °C (77 °F) for no more than 7 days prior to administration. Do not expose unrefrigerated product to temperatures above 25 °C (77 °F). VIVITROL should not be frozen.
Parenteral products should be visually inspected for particulate matter and discoloration prior to administration. |
|
 |
|
 |
Inject the 3.4 mL of diluent into the VIVITROL microsphere vial. (see Figure C) |
 |
Mix the powder and diluent by vigorously shaking the vial for approximately 1 minute. (see Figure D) Ensure that the dose is thoroughly suspended prior to proceeding to Step E. A PROPERLY MIXED SUSPENSION WILL BE MILKY WHITE, WILL NOT CONTAIN CLUMPS, AND WILL MOVE FREELY DOWN THE WALLS OF THE VIAL. |
 |
|
 |
|
 |
|
 |
|
 |
After the injection is administered, cover the needle by pressing the needle protection device against a flat surface using a one-handed technique to activate the safety mechanism away from self and others. (see Figure I) |
 |
Visually confirm needle is fully engaged into the needle protection device. (see Figure J) DISPOSE OF USED AND UNUSED ITEMS IN PROPER WASTE CONTAINERS. |
Frequently asked questions
- What happens if you drink alcohol while taking naltrexone?
- What to avoid when taking naltrexone?
- Should I take naltrexone in the morning or at night?
- How long does naltrexone take to work?
- Can I take Xanax while on Vivitrol?
- Is naltrexone a controlled substance?
- How fast does Vivitrol work?
- How and where is the Vivitrol injection given?
- Does Vivitrol help with cravings?
More about Vivitrol (naltrexone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (203)
- Drug images
- Latest FDA alerts (3)
- Side effects
- Patient tips
- During pregnancy
- Support group
- FDA approval history
- Drug class: drugs used in alcohol dependence
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.