Subsys Dosage
Generic name: FENTANYL 0.1mg
Dosage form: sublingual spray
Drug class: Opioids (narcotic analgesics)
Medically reviewed by Drugs.com. Last updated on Apr 18, 2024.
Important Dosage and Administration Instructions
- Healthcare professionals who prescribe SUBSYS for outpatients must enroll in the TIRF REMS and comply with the requirements of the REMS to ensure safe use of SUBSYS.
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals.
- It is important to minimize the number of strengths available to patients at any time to prevent confusion and possible overdose.
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse .
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with SUBSYS and adjust the dosage accordingly.
- Instruct patients and caregivers to take steps to store SUBSYS securely and to properly dispose of unused SUBSYS as soon as no longer needed.
- Other TIRF formulations and SUBSYS are not equivalent. DO NOT substitute a SUBSYS prescription for any other TIRF formulation under any circumstances. Do not convert patients on a mcg per mcg basis from any other fentanyl product to SUBSYS
- SUBSYS is not bioequivalent with other fentanyl products. Do not convert patients on a mcg per mcg basis from other fentanyl products. There are no conversion directions available for patients on any other fentanyl products, other than Actiq. (Note: This includes oral, transdermal, or parenteral formulations of fentanyl.).
- SUBSYS is NOT a generic version of any other oral transmucosal fentanyl product .
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with SUBSYS [ see Warnings and Precautions ( 5.1) , Patient Counseling Information ( 17) ].
Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Consider prescribing naloxone, based on the patient’s risk factors for overdose, such as concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose. The presence of risk factors for overdose should not prevent the proper management of pain in any given patient [ see Warnings and Precautions ( 5.1, 5.4, 5.6) ].
Consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or overdose.
Initial Dosage
Initiate treatment with SUBSYS for all patients (including those switching from another fentanyl product) using ONE 100 mcg spray sublingually.
Prescribe an initial titration supply of 100 mcg SUBSYS units, which limits the number of units in the home during titration.
Avoid prescribing a higher dose until patients have used up all units to prevent confusion and possible overdose.
Conversion from Actiq to SUBSYS
The initial dose of SUBSYS is always 100 mcg with the only exception of patients already using Actiq.
- For patients being converted from Actiq, prescribers must use the Initial Dosing Recommendations for Patients on Actiq table below ( Table 1). Patients must be instructed to stop the use of Actiq and dispose of any remaining units.
Table 1. Initial Dosing Recommendations for Patients on ACTIQ Current ACTIQ
Dose
(mcg)Initial SUBSYS Dose
(mcg)200 100 mcg spray 400 100 mcg spray 600 200 mcg spray 800 200 mcg spray 1200 400 mcg spray 1600 400 mcg spray - For patients converting from Actiq doses 400 mcg and below, titration should be initiated with 100 mcg SUBSYS and should proceed using multiples of this strength.
- For patients converting from Actiq doses of 600 and 800 mcg, titration should be initiated with 200 mcg SUBSYS and should proceed using multiples of this strength.
- For patients converting from Actiq doses of 1200 and 1600 mcg, titration should be initiated with 400 mcg SUBSYS and should proceed using multiples of this strength.
Dosage Modifications in Patients with Oral Mucositis
In cancer patients with mucositis, exposure to SUBSYS was greater than in patients without mucositis. For patients with Grade 1 mucositis, the increased maximum serum concentration and overall exposure requires closer monitoring for respiratory depression and central nervous system depression, particularly during initiation of therapy with SUBSYS. For patients with Grade 2 mucositis or higher, avoid use of SUBSYS unless the benefits outweigh the potential risk of respiratory depression from increased exposure.
Titration and Maintenance of Therapy
Individually titrate SUBSYS to a dose that provides adequate analgesia and minimizes adverse reactions.
- From the 100 mcg initial dose, closely follow patients and change the dosage level until the patient reaches a dose that provides adequate analgesia using a single SUBSYS dose per breakthrough cancer pain episode with tolerable side effects. Patients should record their use of SUBSYS over several episodes of breakthrough cancer pain and review their experience with their physicians to determine if a dosage adjustment is warranted.
- For each breakthrough pain episode treated, if pain is not relieved after 30 minutes, patients may take ONLY ONE additional dose of the same strength for that episode. Thus patients should take a maximum of two doses of SUBSYS for any breakthrough pain episode.
- Patients MUST wait at least 4 hours before treating another episode of breakthrough pain with SUBSYS.
- If there is a need to titrate to a 200 mcg dose, prescribe 200 mcg SUBSYS units.
- Subsequent titration steps are 400 mcg, 600 mcg, 800 mcg, 1200 mcg and 1600 mcg. See Table 2.
- To reduce the risk of overdose during titration, patients should have only one strength of SUBSYS available at any time.
| SUBSYS DOSE | Using |
| 100 mcg | 1 × 100 mcg unit |
| 200 mcg | 1 × 200 mcg unit |
| 400 mcg | 1 × 400 mcg unit |
| 600 mcg | 1 × 600 mcg unit |
| 800 mcg | 1 × 800 mcg unit |
| 1200 mcg | 2 × 600 mcg unit |
| 1600 mcg | 2 × 800 mcg unit |
SUBSYS Titration Process
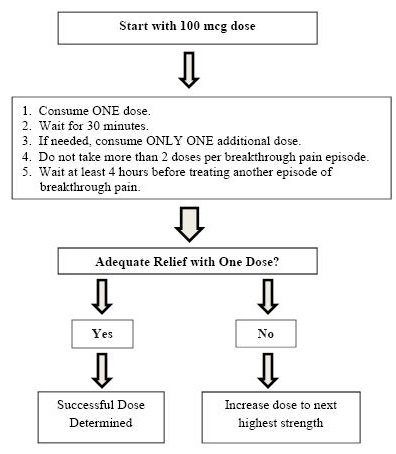
Once titrated to a dose that provides adequate pain relief and tolerable side effects, patients should generally use ONLY ONE SUBSYS dose of the appropriate strength per breakthrough pain episode.
On those occasions when the breakthrough pain episode is not relieved within 30 minutes after administration of the SUBSYS dose, the patient may take ONLY ONE additional dose using the same strength for that episode.
Patients MUST wait at least 4 hours before treating another episode of breakthrough pain with SUBSYS. Once a successful dose has been found, patients should limit consumption to four or fewer doses per day.
Dosage adjustment of SUBSYS may be required in some patients in order to continue to provide adequate relief of breakthrough pain.
If signs of excessive opioid effects appear following administration of a single SUBSYS dose, subsequent doses should be decreased.
Generally, only increase the SUBSYS dose when a single administration of the current dose fails to adequately treat the breakthrough pain episode for several consecutive episodes.
If the patient experiences greater than four breakthrough pain episodes per day, the dose of the maintenance (around-the-clock) opioid used for persistent pain should be re-evaluated. In addition, if pain worsens, re-evaluate the patient for changes in the underlying pain condition.
Discontinuation of SUBSYS
For patients no longer requiring opioid therapy, consider discontinuing SUBSYS along with a gradual downward titration of other opioids to minimize possible withdrawal effects. In patients who continue to take their chronic opioid therapy for persistent pain but no longer require treatment for breakthrough pain, SUBSYS therapy can usually be discontinued immediately. [ see Drug Abuse and Dependence ( 9.3) ].
Disposal of SUBSYS
Patients and caregivers must be advised to dispose of used unit dose systems immediately after use and any unneeded unit dose systems remaining from a prescription as soon as they are no longer needed. Consumed units represent a special risk because they are no longer protected by the child resistant blister package, yet may contain enough medicine to be fatal to a child..
Charcoal-lined disposal pouches are provided with every carton dispensed. A charcoal-lined disposal pouch is to be used by patients or their caregivers to dispose of the contents of any unneeded unit dose systems when they are no longer needed. Instructions for usage of the charcoal-lined disposal pouch are included in the Medication Guide and Instructions for Use.
Frequently asked questions
- Which drugs cause opioid-induced constipation?
- How long does Fentanyl stay in your system?
- Why is fentanyl so dangerous?
- Carfentanil vs Fentanyl: Which is more dangerous?
- Fentanyl test strips: where to get & how to use?
- Which painkiller should you use?
- What are the symptoms of a fentanyl overdose?
- How does fentanyl compare to heroin or other opiates?
More about Subsys (fentanyl)
- Check interactions
- Compare alternatives
- Reviews (12)
- Latest FDA alerts (14)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: Opioids (narcotic analgesics)
- Breastfeeding
- En español
Patient resources
Other brands
Duragesic, Sublimaze, Actiq, Fentora, ... +4 more
Professional resources
Other brands
Duragesic, Sublimaze, Actiq, Fentora, ... +2 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

