Oxervate Dosage
Generic name: CENEGERMIN 20ug in 1mL
Dosage form: ophthalmic solution
Drug class: Miscellaneous ophthalmic agents
Medically reviewed by Drugs.com. Last updated on Feb 5, 2025.
General Dosing Information
Contact lenses should be removed before applying OXERVATE and may be reinserted 15 minutes after administration.
If a dose is missed, treatment should be continued as normal, at the next scheduled administration.
If more than one topical ophthalmic product is being used, administer the eye drops at least 15 minutes apart to avoid diluting products. Administer OXERVATE 15 minutes prior to using any eye ointment, gel or other viscous eye drops.
Recommended Dosage and Dose Administration
Instill one drop of OXERVATE in the affected eye(s), 6 times a day at 2-hour intervals for eight weeks.
Preparation for Administration
Remove the weekly carton(s) containing OXERVATE vials from the insulated pack and store it for up to 14 days in a refrigerator (no later than 5 hours from when you receive the medicine from your pharmacy). OXERVATE is stored in a freezer at the pharmacy. If treatment is started immediately after receiving the weekly carton, wait until the first vial is thawed (this could take up to 30 minutes when kept at room temperature up to 77°F (25°C)). Do not shake the vial.
Follow Steps 1 to 19 each day you use OXERVATE:
Take an individual vial of OXERVATE from the refrigerator in the morning and prepare it in the following way:
|
Step 1. Wash your hands. Step 2. If you wear contact lenses, take them out before using OXERVATE. |
|
|
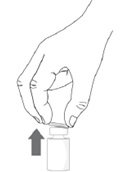
Step 3. Remove the plastic flip-off cap from the vial. |
 |
|
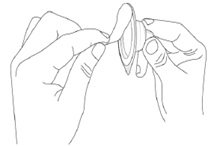
Step 4. Peel-off the back of the vial adapter blister pack. |
 |
|
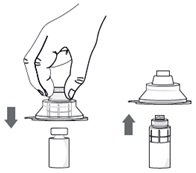
Step 5. Without removing the vial adapter from its blister pack, connect it to the vial by firmly pushing it down until it snaps into place over the neck of the vial. The spike of the vial adapter should pierce through the vial’s rubber stopper. After the vial adapter has been connected correctly, do not remove it from the vial. |
 |
The multi-dose vial of OXERVATE is now ready for use (1 drop in the affected eye every 2 hours six times a day).
To withdraw and give each dose of OXERVATE, follow the Steps 7 to 19:
|
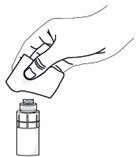
Step 7. Take a single sterile disinfectant wipe and gently clean the surface of the valve on the connector part of the vial adapter. |
 |
|
Step 8. Remove a pipette from its protective packaging. |
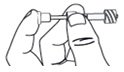 |
|
Step 9. Screw the pipette (clockwise) into the connector part of the vial adapter. Step 10. Make sure that the pipette plunger is pushed all the way down. |
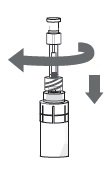 |
|
Step 11. Turn the vial upside-down with the pipette still connected. Gently pull the plunger until it stops, to draw the eye drop solution into the pipette. Make sure the plunger has reached the stop point. Step 12. Check the pipette to make sure it contains the eye drop solution. Air bubbles may cause blockage and prevent the pipette from filling properly (especially the first time you withdraw the eye drop solution). If the pipette is empty, keep the vial with the connected pipette upside-down, push the plunger all the way in and pull it out again. |
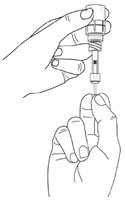 |
|
Step 13. After the pipette has been correctly filled, unscrew the pipette from the connector part of the vial adapter (counter-clockwise). Pull the pipette straight up to remove it. |
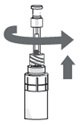 |
|
Step 14.
|
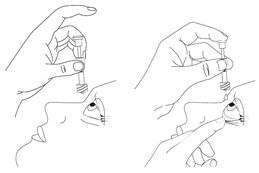 |
|
Step 15. Throw away the used pipette right away after use, even if there is still some eye drop solution left in it. Only use 1 pipette for each eye and each dose. If you miss your eye and there is no longer any eye drop solution in the pipette, try again, using a new pipette and wipe (See Steps 7 to 14). |
|
|
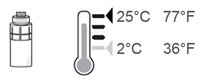
Step 16. After each use throughout the day, place the vial back in the refrigerator or keep it below 77°F (25°C) for the rest of the day, with the vial adapter still connected. |
 |
|
Step 17. Repeat from Step 7 to Step 16 every 2 hours 6 times a day, using a new sterile disinfectant wipe and a new pipette each time. If you use drops in both eyes, repeat the above instructions for your other eye using a new pipette. You will need to use 2 vials each day. Store the vial below 77°F (25°C) throughout the day. You can also store the vial in the refrigerator but do not freeze the vial. |
|
|
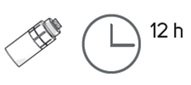
Step 18. Throw away the used vial at the end of each day even if there is still some eye drop solution left in it. Throw away the vial no later than 12 hours from the time you connected the vial adapter to it even if there is eye solution still left in the vial. |
 |
|
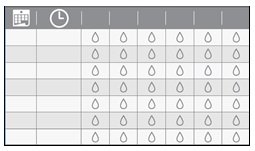
Step 19. Track each time you instill an eye drop of OXERVATE on the weekly Dose Recording Card provided with the delivery system. This will allow you to track your 6 doses each treatment day, the date of the first use of the weekly supply and the time of the vial opening (which is when you connect the vial adapter to the vial) during the week. |
 |
To make sure accurate dosing every 2 hours, you may want to set an alarm as a reminder for dosing.
Frequently asked questions
More about Oxervate (cenegermin ophthalmic)
- Compare alternatives
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous ophthalmic agents
- Breastfeeding
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

