Menveo Dosage
Generic name: NEISSERIA MENINGITIDIS GROUP A CAPSULAR OLIGOSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 10ug in 0.5mL; NEISSERIA MENINGITIDIS GROUP C CAPSULAR OLIGOSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 5ug in 0.5mL, NEISSERIA MENINGITIDIS GROUP W-135 CAPSULAR OLIGOSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 5ug in 0.5mL, NEISSERIA MENINGITIDIS GROUP Y CAPSULAR OLIGOSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 5ug in 0.5mL
Dosage form: injection
Drug class: Bacterial vaccines
Medically reviewed by Drugs.com. Last updated on Mar 31, 2025.
For intramuscular injection only.
MENVEO Presentations
MENVEO is supplied in two presentations, a two-vial presentation and a one-vial presentation.
Two-Vial Presentation
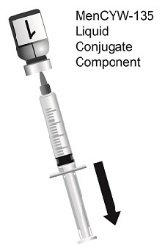
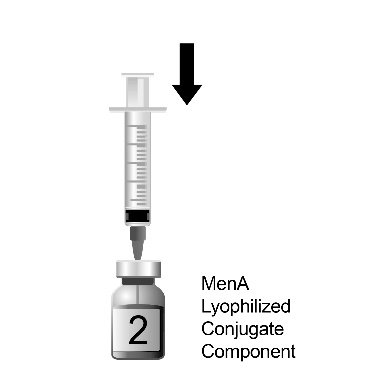
The two-vial presentation includes a vial with a gray cap containing the MenCYW-135 liquid conjugate component and a vial with an orange cap containing the MenA lyophilized conjugate component. The contents of the vials must be combined to form MENVEO prior to administration. This presentation is for use in individuals 2 months through 55 years of age.
One-Vial Presentation
The one-vial presentation contains MENVEO in a single vial with a pink cap and does not require reconstitution before use. This presentation is for use in individuals 10 through 55 years of age.
Preparation
Reconstitution Instructions for MENVEO Two-Vial Presentation
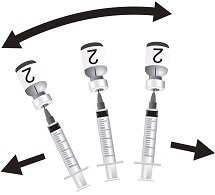
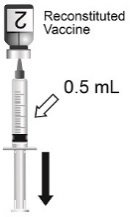
Use the MenCYW-135 liquid conjugate component (Vial 1, gray cap) to reconstitute the MenA lyophilized conjugate component (Vial 2, orange cap) to form MENVEO. Invert Vial 2 and shake well until the lyophilized conjugate component is dissolved. After reconstitution, withdraw 0.5 mL from the vial containing the reconstituted vaccine. See Figures 1 through 4.
Administer MENVEO immediately after reconstitution.
Instructions for MENVEO One-Vial Presentation
The MENVEO presentation that is supplied in a single vial with a pink cap does NOT require reconstitution. Withdraw 0.5 mL from the vial.
Administration
MENVEO is a clear, colorless solution, free from visible foreign particles. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If any of these conditions exist, MENVEO should not be administered.
Administer a single 0.5-mL dose by intramuscular injection.
Dosing Schedule
The dosing schedule is as follows:
Primary Vaccination
| MENVEO Two-Vial Presentation | |
|---|---|
|
Infants Aged 2 Months |
4-dose series at 2, 4, 6, and 12 months of age |
|
Children Aged 7 through 23 Months |
2-dose series with the second dose administered in the second year of life and at least 3 months after the first dose |
|
Children Aged 2 through 10 Years |
A single dose For children aged 2 through 5 years at continued high risk of meningococcal disease, a second dose may be administered 2 months after the first dose. |
|
Adolescents and Adults Aged 11 through 55 Years |
A single dose |
|
MENVEO One-Vial Presentation |
|
|
Adolescents and Adults Aged 10 through 55 Years |
A single dose |
Booster Vaccination
Adolescents and Adults Aged 15 through 55 Years: A single booster dose of MENVEO using either the two-vial presentation or the one-vial presentation may be administered to individuals who are at continued risk for meningococcal disease if at least 4 years have elapsed since a prior dose of a meningococcal (serogroups A, C, Y, W-135) conjugate vaccine.
More about Menveo (meningococcal conjugate vaccine)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- FDA approval history
- Drug class: bacterial vaccines
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.