Lutrate Depot Dosage
Generic name: LEUPROLIDE ACETATE 22.5mg in 2mL;
Dosage form: injection
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
Medically reviewed by Drugs.com. Last updated on Mar 14, 2025.
LUTRATE DEPOT 22.5 mg for 3-Month Administration
LUTRATE DEPOT must be administered under the supervision of a physician.
In patients treated with GnRH analogues for prostate cancer, treatment is usually continued upon development of metastatic castration-resistant prostate cancer.
| Dosage | 22.5 mg for 3-Month Administration |
| Recommended Dose | 1 injection every 12 weeks |
The recommended dose of LUTRATE DEPOT 22.5 mg for 3-month administration is one injection every 12 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and administered every 12 weeks as a single intramuscular injection.
Reconstitution Instructions for LUTRATE DEPOT
- Reconstitute and administer the lyophilized microspheres as a single intramuscular injection.
- The suspension should be administered immediately after reconstitution.
- As with other drugs administered by intramuscular injection, the injection site should be alternated periodically.
- Visually inspect LUTRATE DEPOT powder (white to off-white powder). DO NOT USE the vial if clumping or caking is evident. A thin layer of powder on the wall of the vial is considered normal prior to mixing with the diluent. The diluent contained in the prefilled syringe should appear clear and colorless.
- Use ONLY the provided diluent for reconstitution of LUTRATE DEPOT. DO NOT use other diluents.
- The reconstituted product is a suspension of milky, white color appearance.
LUTRATE DEPOT is packaged in a commercial kit. Each kit contains:
- One vial containing 22.5 mg of leuprolide acetate as lyophilized microspheres.
- One prefilled syringe containing 2 mL of mannitol for injection.
- One MIXJECT transfer device including one needle.

Please read the instructions completely before you begin.
MIXJECT Preparation
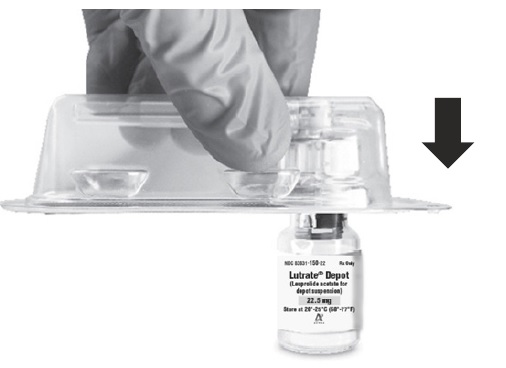
Wash your hands with soap and hot water and put on gloves1 immediately prior to preparing the injection. Place the tray on a clean, flat surface that is covered with a sterile pad or cloth. Remove the MIXJECT device, the backstop, the prefilled syringe containing the solvent for reconstitution and the LUTRATE DEPOT vial.
Remove the Flip-Off button from the top of the vial, revealing the rubber stopper. Place the vial in a standing upright position on the prepared surface. Disinfect the rubber stopper with the alcohol wipe. Discard the alcohol wipe and allow the stopper to dry. Insert the backstop to the flange of the syringe until you feel it snap in place. Proceed to MIXJECT Activation.

MIXJECT Activation
- Peel the cover away from the blister pack containing the vial adapter (MIXJECT). Do not remove the vial adapter from the blister pack. Place the blister pack containing the vial adapter firmly on the vial top, piercing the vial. Push down gently until you feel it snap in place.
- Remove the cap from the syringe barrel and then, remove the blister pack from the vial adapter. Connect the syringe to the vial adapter by screwing it clockwise into the opening on the side of the vial adapter. Be sure to gently twist the syringe until it stops turning to ensure a tight connection.
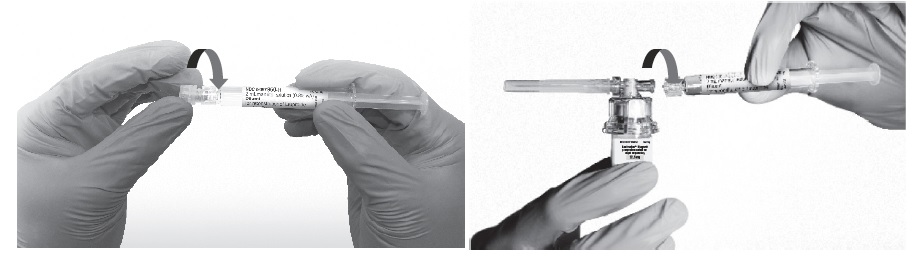
- While holding the vial, place your thumb on the plunger rod and push the plunger rod in all the way to transfer the diluent from the pre-filled syringe into the vial. Do not release the plunger rod.
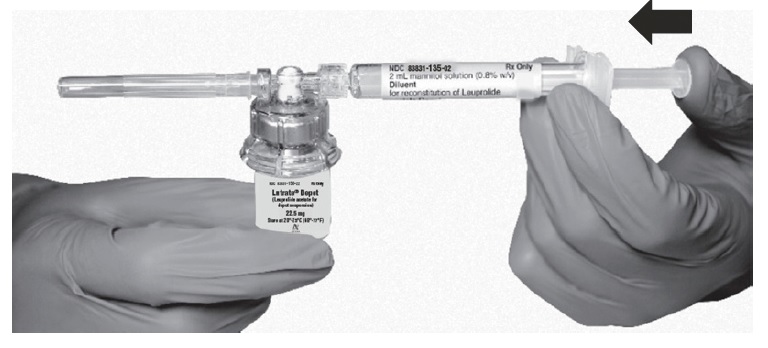
- Keeping the plunger rod depressed, gently swirl the vial for approximately one minute until a uniform milky-white suspension is obtained. This will ensure complete mixing of LUTRATE DEPOT and the sterile mannitol solution diluent. The suspension will now have a milky appearance. In order to avoid separation of the suspension, proceed to the next steps without delay.
- Invert the MIXJECT system so that the vial is at the top. Grasp the MIXJECT system firmly by the syringe and pull back the plunger rod slowly to draw the reconstituted LUTRATE DEPOT into the syringe.
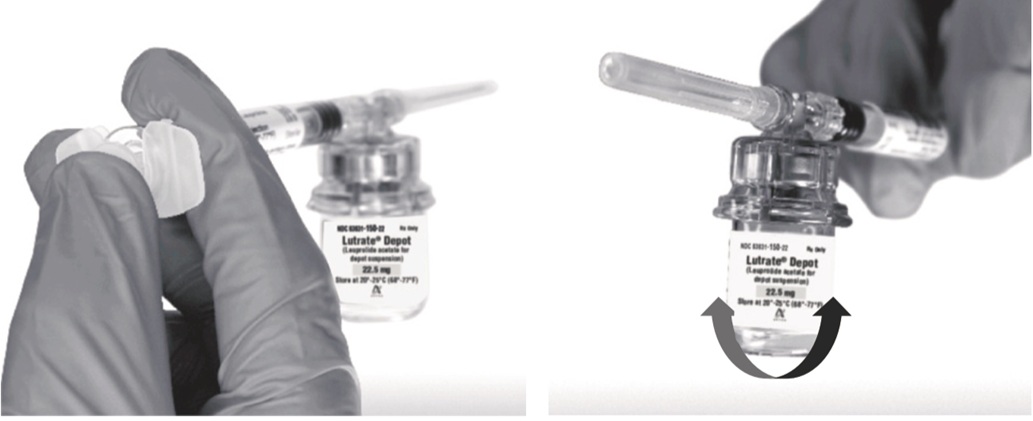
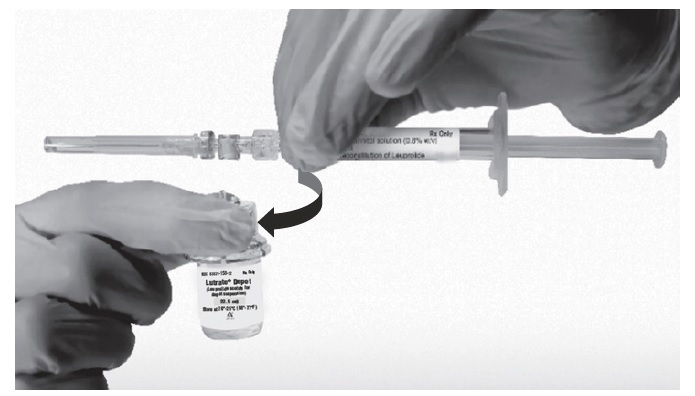
Return the vial to its upright position, and disconnect the vial adapter from the MIXJECT syringe assembly by grabbing firmly the syringe and turning the plastic cap of the vial adapter clockwise. Grasp only the plastic cap when removing.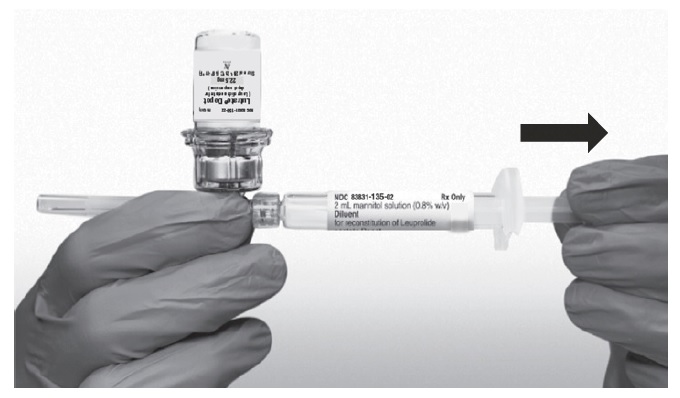
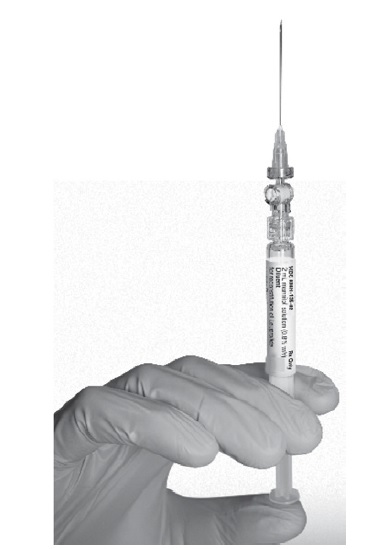
- Keep the syringe UPRIGHT. With the opposite hand pull the needle cap upward. Advance the plunger to expel the air from the syringe. The syringe containing LUTRATE DEPOT is now ready for administration. The suspension should be administered immediately after reconstitution.
- After cleaning the injection site with an alcohol swab, administer the intramuscular injection by inserting the needle at a 90 degree angle into the gluteal area, anterior thigh, or deltoid; injection sites should be alternated (see Figure).
NOTE: If a blood vessel is accidentally penetrated, aspirated blood will be visible just below the luer lock. If blood is present, remove the needle immediately. Do not inject the medication.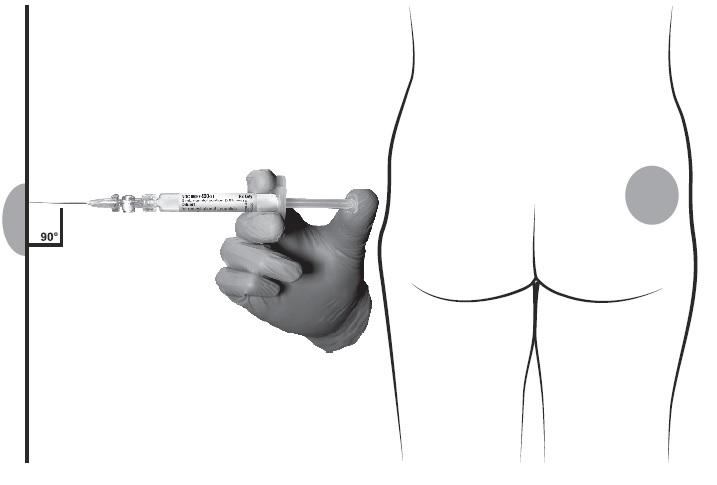
- Inject the entire contents of the syringe intramuscularly.
- Withdraw the needle. Once the syringe has been withdrawn, immediately discard the needle into a suitable sharps container. Dispose the syringe according to local regulations/procedures.
Frequently asked questions
- Will I get my period while on Lupron?
- What does Lupron do for IVF?
- Are Lupron Depot and Eligard the same drug?
- Can you get pregnant on Lupron Depot?
- Is Firmagon (degarelix) the same as Lupron?
More about Lutrate Depot (leuprolide)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- Drug class: gonadotropin releasing hormones
Patient resources
Other brands
Eligard, Lupron Depot, Camcevi, Fensolvi, Viadur
Professional resources
Other brands
Eligard, Lupron Depot, Camcevi, Fensolvi, ... +2 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.









