Kebilidi Dosage
Generic name: ELADOCAGENE EXUPARVOVEC 560000000000{GC} in 1mL
Dosage form: suspension, for intraputaminal infusion
Medically reviewed by Drugs.com. Last updated on Nov 27, 2024.
For single-dose intraputaminal infusion only.
2.1 Important Dosing Information
- Confirm patient has AADC deficiency due to biallelic mutations in the DDC gene.
- Strictly observe aseptic technique during preparation and administration of KEBILIDI.
- KEBILIDI should be administered in a medical center which specializes in stereotactic neurosurgery.
- Administer KEBILIDI only using an FDA-authorized cannula for intraparenchymal infusion (i.e., ClearPoint SmartFlow Neuro Cannula Part Number NGS-NC-01-EE or NGS-NC-02-EE).
- Use of the syringe (i.e., connecting the syringe to the syringe pump and priming of the cannula) should begin within 6 hours of starting product thaw
- KEBILIDI is intended to be administered with an infusion pump capable of infusing at a rate of 0.003 mL/min.
2.2 Recommended Dose
KEBILIDI is administered as four intraputaminal infusions in a single stereotactic neurosurgical procedure as per the recommended dose shown in Table 1.
Table 1: Recommended Dose of KEBILIDI
| Total Recommended Dose | 1.8x1011 vg (0.32 mL) |
| Total number of infusions | 4 |
| Volume (dose) per infusion | 0.08 mL (0.45x1011 vg) |
| Location of infusions | 2 in anterior putamen, 2 in posterior putamen |
| Infusion rate at each target point | 0.003 mL/min |
| Dose duration for infusion at each target point | 27 minutes |
2.3 Preparation
Thawing KEBILIDI Vial
- Coordinate timing of KEBILIDI thaw and infusion. KEBILIDI should be used within 6 hours of starting product thaw. Infusion of KEBILIDI takes 4 hours. The maximum time from thaw to completion of infusion should be no more than 10 hours.
- Thaw the KEBILIDI vial upright at room temperature before use. The contents of the vial will thaw in approximately 15 minutes at room temperature. Do not thaw or warm the vial any other way. Gently invert the vial 3 times. Do not shake the vial.
- Inspect the fully thawed KEBILIDI vial after mixing. KEBILIDI should be inspected visually for particulate matter, and discoloration prior to administration. KEBILIDI is clear to slightly opaque. The color of KEBILIDI should be a colorless to faint white suspension
- Do not use if particulates, or discoloration are visible in the suspension.
Preparing KEBILIDI in Syringe
- Gather supplies listed in Table 2 for preparation:
Table 2: Supplies for KEBILIDI PreparationAbbreviations: PC=Polycarbonate; PP=Polypropylene Component Material of Construction 1mL lubricated sterile Luer-lock syringe with elastomer plunger
Or
5mL lubricated sterile Luer-lock syringe with elastomer plungerSilicone, PC; Silicone, PP
Silicone, PP18 or 19 G sterile needle with 5µm filter Stainless steel, PC hub; Stainless steel, PP hub Sterile Luer-lock syringe cap - Plastic bag for delivery into surgical unit - Secondary container for delivery into surgical unit - - Prepare KEBILIDI using sterile techniques under aseptic conditions, proper engineering controls (e.g., biological safety cabinets or isolator) as per the institutional policies.
- Open the syringe and label it as the product-filled syringe.
- Attach the filter needle to the syringe.
- Draw the full volume of the vial of KEBILIDI into the syringe. Invert the vial and syringe and partially withdraw or angle the needle as necessary to maximize recovery of product.
- Draw air into the syringe so that the needle is emptied of product. Carefully remove the needle from syringe containing KEBILIDI. Purge the air from the syringe until there is no air bubble and then cap with a syringe cap.
- Place the syringe in a plastic bag and seal the bag.
- Place the plastic bag in an appropriate secondary container for delivery to the surgical suite at room temperature.
- The filled syringe prepared under aseptic conditions for delivery to the surgical site should be used immediately.
Notes:
- Do not refreeze thawed product.
- Dispose any remaining KEBILIDI or disposable material in compliance with institutional policy
2.4 Administration
Gather supplies for administration:
- KEBILIDI
- SmartFlow Neuro Cannula
- Syringe pump, capable of an infusion rate of 0.003 mL/min and compatible with 1 mL or 5 mL syringe sizes
- Stereotactic system
Identification of the Target Points Within the Putamen
- Using standard neurosurgical stereotactic procedure, brain imaging for stereotactic planning and intraoperative navigation should be done prior to the procedure (see Figure 1).
Figure 1: Four Target Points within the Putamen for Infusion Sites
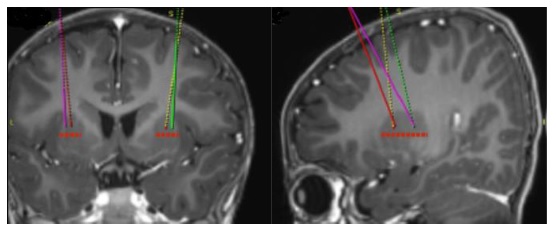
- After stereotactic registration is complete, mark the entry point on the skull. Surgical access through the skull bone and dura should be performed.
Figure 2: Infusion Delivery System
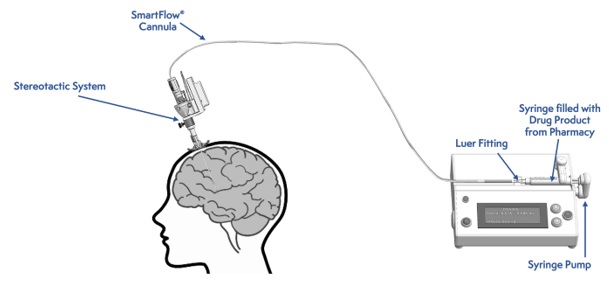
Intraputaminal Administration of KEBILIDI
Administer KEBILIDI by bilateral intraputaminal infusion using a single infusion cannula in one surgical session at two sites (anterior and posterior) per putamen. The infusion cannula is placed and infusion performed separately for each target point (see Figure 2).
- Tightly connect the syringe containing the prepared KEBILIDI to Luer Lock connector at the proximal end of the infusion cannula.
- Load syringe onto the infusion pump and secure appropriately.
- Set infusion pump occlusion limit to the highest level to prevent pump from alarming or disrupting the infusion.
- Prime KEBILIDI at the rate of up to 0.003 mL/minute (0.18 mL/hour) until the first drop of the product can be seen at the tip of the needle.
- Place sterile absorbent pad or gauze under the tip of the cannula to contain drops of the prepared product that might emerge during priming.
- Run the infusion pump prior to insertion of the cannula to ensure the prepared product is flowing from the tip immediately before insertion.
- Place the infusion SmartFlow Neuro Cannula at the designation point in the putamen using stereotactic tools based on pre-planned stereotactic trajectories.
- Starting with the first target site, insert the cannula through a burr hole into the putamen and then incrementally withdraw cannula along the intraputaminal infusion track, distributing the 0.08 mL (infused at a rate of 0.003 mL/min) of KEBILIDI per putamen across the planned trajectory to optimize distribution across the target site. The pump should run continuously throughout the 27-minute infusion, including during the repositioning to the designated sites along the infusion track.
- Once the infusion is complete, stop the pump and leave the cannula in place for 5 minutes before withdrawing. Re-zero the total delivered volume setting on the infusion pump as soon as the cannula is inserted to the target and perform infusion. Reinsert at the next target point, repeating the same procedure for the other 3 target points.
- After standard neurosurgical closure procedures, carry out a postoperative brain imaging examination of the patient to ensure there are no complications (e.g., bleeding).
More about Kebilidi (eladocagene exuparvovec)
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.



