Ixiaro Dosage
Generic name: JAPANESE ENCEPHALITIS VIRUS STRAIN SA 14-14-2 ANTIGEN (FORMALDEHYDE INACTIVATED) 6[arb'U] in 0.5mL
Dosage form: injection, suspension
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Nov 25, 2024.
For intramuscular administration only.
Dosage and Schedule
Primary Series:
Children 2 months to <3 years of age: Primary immunization with IXIARO consists of two (2) 0.25 mL doses, administered 28 days apart.
Individuals 3 years of age and older: Primary immunization with IXIARO consists of two (2) 0.5 mL doses, administered 28 days apart.
Complete the primary immunization series at least 1 week prior to potential exposure to JEV.
Booster Dose:
Individuals 17 years of age and older: If the primary series of two doses was completed more than 1 year previously, a booster dose may be given if ongoing exposure or re-exposure to JEV is expected.
Infants, children and adolescents 2 months to <17 years of age: The safety and immunogenicity of a booster dose has not been evaluated.
Administration
IXIARO is administered intramuscularly. The preferred sites for intramuscular injection are the anterolateral aspect of the thigh in infants 2 to 11 months of age, the anterolateral aspect of the thigh (or the deltoid muscle if muscle mass is adequate) in children 1 to <3 years of age, or the deltoid muscle in individuals 3 years of age and older.
Do not administer intravenously, intradermally, or subcutaneously.
Preparation for Administration
Prior to agitation, IXIARO is a clear liquid with a white precipitate. Before administration, shake the syringe well to obtain a white, opaque, homogeneous suspension.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If either of these conditions exists, do not administer.
Preparation of a 0.25 mL Dose of IXIARO for Administration to Children 2 Months to <3 Years of Age:
1. Shake the pre-filled syringe containing 0.5 mL to obtain a homogeneous suspension.
2. Remove the syringe tip cap by gently twisting it. Do not attempt to snap or pull the tip off as this may damage the syringe.
3. Attach a sterile safety needle to the pre-filled syringe (needle is not provided with IXIARO).
4. Hold the syringe in an upright position and uncap the needle.
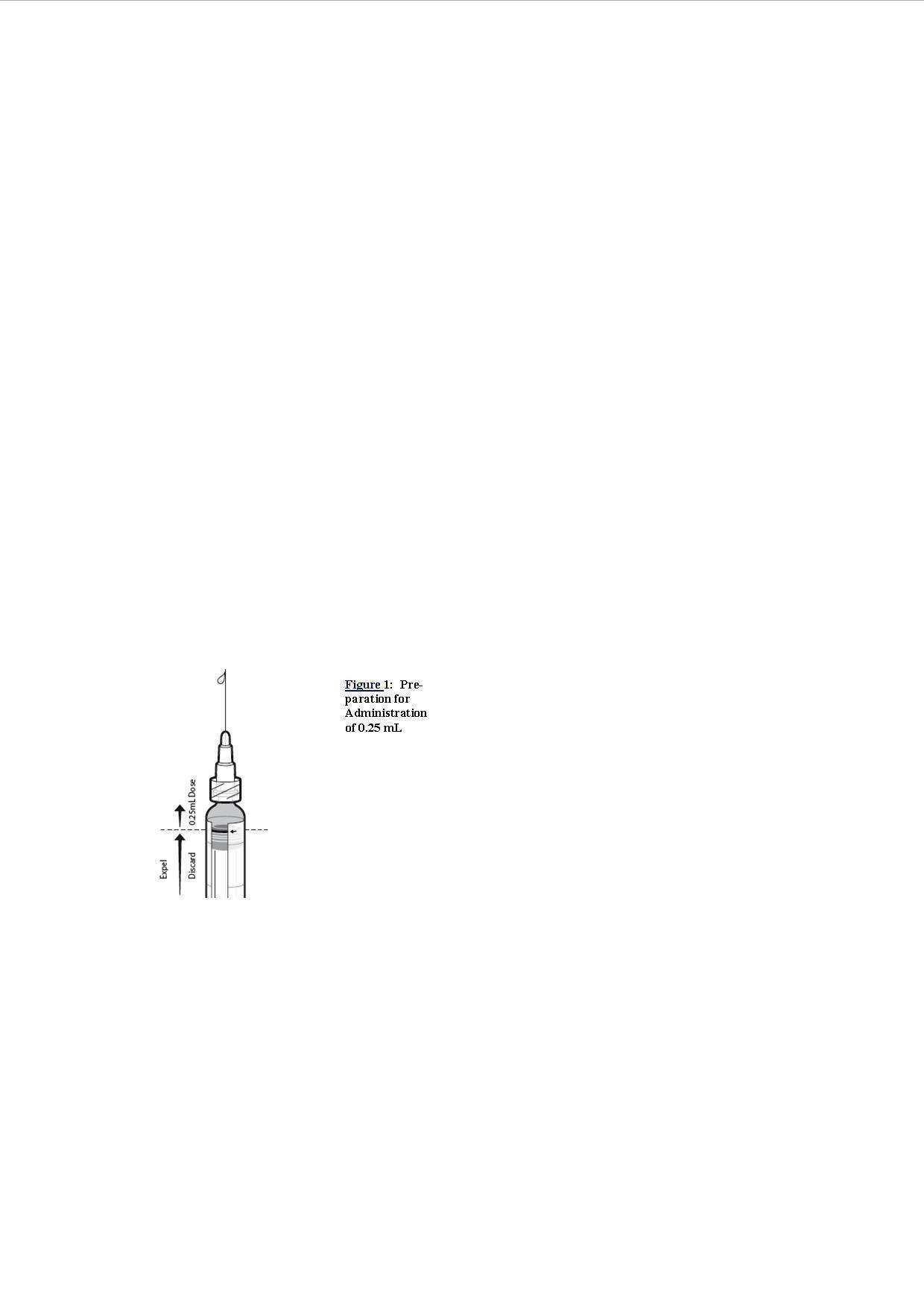
5. Push the plunger stopper up to the edge of the red line on the syringe barrel, indicated by a red arrow (see Figure1)*, and discard expelled volume into a medical waste container.
6. Lock the needle safety shield and remove the needle.
7. Attach a new sterile needle prior to injection of the remaining volume.
*If the plunger stopper is pushed beyond the red line, do not administer the vaccine. Repeat the procedure using a new pre-filled syringe.
Preparation of a 0.5 mL Dose of IXIARO for Administration to Individuals 3 Years of Age and Above:
1. Shake the pre-filled syringe containing 0.5 mL to obtain a homogeneous suspension.
2. Remove the syringe tip cap by gently twisting it. Do not attempt to snap or pull the tip off as this may damage the syringe.
3. Attach a sterile needle to the pre-filled syringe (needle is not provided with IXIARO).
More about Ixiaro (japanese enceph vacc sa14-14-2, inactivated)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: viral vaccines
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
