Imovax Rabies Dosage
Generic name: RABIES VIRUS STRAIN PM-1503-3M ANTIGEN (PROPIOLACTONE INACTIVATED) 2.5[iU] in 1mL;
Dosage form: intramuscular injection kit
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Aug 12, 2025.
For intramuscular use
Pre-exposure Prophylaxis Dose and Schedule
Each dose of IMOVAX RABIES is 1 mL.
Primary Vaccination series:
- Three-dose schedule: Administer a dose at Day 0, 7, and 21 or 28
OR - Two-dose schedule: Administer a dose at Day 0 and 7
Booster:
Administer a single dose of IMOVAX RABIES.
The United States Centers for Disease Control and Prevention (CDC) has published clinical guidance on administration of booster doses of rabies vaccine depending on rabies exposure risk and for vaccination of individuals who are immunocompromised or who are receiving chloroquine or drugs related to chloroquine. (1)
Post-exposure Prophylaxis Dose and Schedule
Each dose of IMOVAX RABIES is 1 mL.
The IMOVAX RABIES post-exposure prophylaxis schedule depends on whether or not the individual has been previously vaccinated and/or immunized against rabies. The CDC considers individuals to be previously vaccinated if they received one of the recommended cell-culture vaccines either in a pre-exposure or post-exposure prophylaxis regimen, or they received another vaccine regimen (or vaccines other than cell-culture vaccine) and had a documented adequate rabies virus neutralizing antibody (RVNA) response (defined as ≥ 0.5 IU/mL by Rapid Fluorescent Focus Inhibition Test [RFFIT]). (2)
Previously Vaccinated Individuals
In previously vaccinated individuals (see definition above),
- Administer a dose of IMOVAX RABIES immediately after exposure (at Day 0) and at Day 3.
Unvaccinated or Immunosuppressed (irrespective of previous vaccination status) Individuals
For individuals who have not previously received rabies vaccine, or who are immunosuppressed,
- Administer a dose of IMOVAX RABIES immediately after exposure (at Day 0) and at Day 3, 7, 14, and 28. Administer each dose of IMOVAX RABIES at an anatomical site distant from the Human Rabies Immunoglobulin (HRIG) administration site(s).
- Administer HRIG at Day 0 according to its Prescribing Information.
- Post-vaccination serologic testing is indicated when the individual is known to be immunosuppressed. (3) Contact local or state health department or CDC for recommendations for serologic testing. (3)
Individuals with Uncertain Immune Status
In individuals whose RVNA status is not known and who 1) have not received the full recommended regimen of a cell culture vaccine either in a pre-exposure or post-exposure prophylaxis regimen OR 2) received another (non-cell culture) rabies vaccine regimen,
- Administer a dose of IMOVAX RABIES immediately after exposure (at Day 0) and at Day 3, 7, 14, and 28. Administer each dose of IMOVAX RABIES at an anatomical site distant from the HRIG administration site(s).
- Administer HRIG at Day 0 according to its Prescribing Information.
- If antibody levels ≥ 0.5 IU/mL by RFFIT can be demonstrated in a serum sample collected before vaccine is given, vaccinations may be discontinued after at least two doses of IMOVAX RABIES. (1) (2)
Preparation
IMOVAX RABIES is supplied in a package containing a vial of lyophilized vaccine, a Luer-Lok prefilled syringe containing 1 mL of Sterile Water diluent with a plunger rod either inserted into the syringe or provided separately, and a sterile reconstitution needle. Reconstitute the lyophilized vaccine with the Sterile Water diluent to form IMOVAX RABIES, as described in the instructions below.
Instructions for vaccine reconstitution
Step 1: Screw the plunger rod into the syringe containing Sterile Water diluent if the plunger rod is provided separately.
| Step 2: Hold the syringe cap (avoid holding the plunger rod or syringe barrel) and unscrew the tip cap by twisting it counterclockwise. | 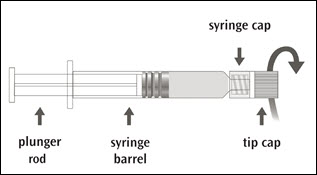 |
| Step 3: Attach the reconstitution needle to the syringe, by gently twisting the needle clockwise into the syringe until slight resistance is felt. | 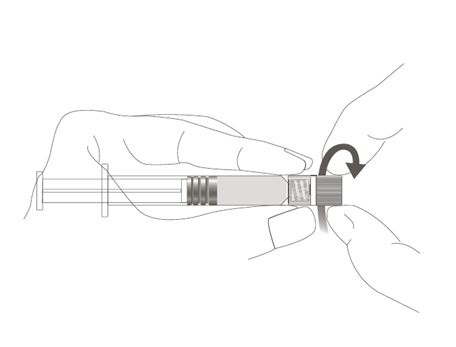 |
Step 4: Transfer the entire content of the syringe containing Sterile Water diluent into the vial containing lyophilized vaccine.
Step 5: Gently swirl the vial until completely dissolved. After reconstitution, IMOVAX RABIES is pink to red.
Step 6: Unscrew the syringe from the reconstitution needle to eliminate negative pressure.
Reattach the syringe to the reconstitution needle which has remained in the vial.
Step 7: Withdraw the total content of the vial (approximately 1 mL) containing reconstituted vaccine. Remove the reconstitution needle and discard it.
Step 8: Attach a new sterile needle (as per Step 3) of a proper length and gauge suitable for intramuscular administration.
Parenteral drug products should be inspected for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, IMOVAX RABIES should not be administered.
Administration
Administer IMOVAX RABIES intramuscularly immediately after reconstitution.
For adults and older children, the vaccine should be injected into the deltoid muscle. (4) (5) (6) In infants and small children, the anterolateral aspect of the thigh may be preferable, depending on age and body mass.
Do not administer in the gluteal area.
Do not administer intradermally.
More about Imovax Rabies (rabies vaccine, human diploid cell)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- Drug class: viral vaccines
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

