Tauramox (moxidectin) Injectable Solution for Beef and Nonlactating Dairy Cattle
This treatment applies to the following species:Antiparasitic
Contains 10 mg moxidectin/mL
Not for use in female dairy cattle 20 months of age or older (including dry dairy cows), veal calves, and calves less than 8 weeks of age.
For Treatment of Infections and Infestations Due to Internal and External Parasites of Cattle
Consult your veterinarian for assistance in the diagnosis, treatment and control of parasitism.
Product Description
Tauramox™ Injectable Solution is a ready-to-use, sterile solution containing 1% moxidectin. Moxidectin is an endectocide in the milbemycin chemical class which shares the distinctive mode of action characteristic of macrocyclic lactones. Tauramox™ Injectable is specially formulated to allow moxidectin to be absorbed from the site of injection and distributed internally to the areas of the body affected by endo- and/or ectoparasitism. Moxidectin binds selectively and with high affinity to glutamate-gated chloride ion channels which are critical to the function of invertebrate nerve and muscle cells. This interferes with neurotransmission resulting in paralysis and elimination of the parasite.
Tauramox (moxidectin) Injectable Solution for Beef and Nonlactating Dairy Cattle Indications
Tauramox™ Injectable, when administered at the recommended dose level of 0.2 mg/2.2 lb (0.2 mg/kg) body weight, is effective in the treatment and control of the following internal and external parasites of cattle:
Gastrointestinal Roundworms
Ostertagia ostertagi - Adults and L4 (including inhibited Larvae)
Haemonchus placei - Adults
Trichostrongylus axei - Adults and L4
Trichostrongylus colubriformis - Adults and L4
Cooperia oncophora - Adults
Cooperia pectinata - Adults
Cooperia punctata - Adults and L4
Cooperia spatulata - Adults
Cooperia surnabada - Adults and L4
Nematodirus helvetianus - Adults
Oesophagostomum radiatum - Adults and L4
Trichuris spp. - Adults
Lungworms
Dictyocaulus viviparus - Adults and L4
Cattle Grubs
Hypoderma bovis
Hypoderma lineatum
Mites
Psoroptes ovis (Psoroptes communis var. bovis)
Lice
Linognathus vituli
Solenopotes capillatus
Persistent Activity: Moxidectin Injectable has been proven to effectively protect cattle from reinfection with Dictyocaulus viviparus and Oesophagostomum radiatum for 42 days after treatment, Haemonchus placei for 35 days after treatment, and Ostertagia ostertagi and Trichostrongylus axei for 14 days after treatment.
Management Considerations for External Parasites: For most effective external parasite control, Tauramox™ Injectable should be administered to all cattle in the herd. Cattle entering the herd following this administration should be treated prior to introduction. Consult your veterinarian or a livestock entomologist for the most appropriate time to administer Tauramox™ Injectable in your location to effectively control external parasites.
Dosage
The recommended rate of administration for Tauramox™ Injectable is 1 mL for each 110 lb (50 kg) body weight to provide 0.2 mg moxidectin/2.2 lb (0.2 mg/kg) body weight. The table below will assist in the calculation of the appropriate volume of injectable which must be administered based on the weight of animal being treated. Be careful not to overdose animals; estimate animal’s body weight as closely as possible or weigh animals individually.
|
Weight (lb) |
165 |
220 |
330 |
440 |
550 |
660 |
770 |
880 |
990 |
1100 |
|
Weight (kg) |
75 |
100 |
150 |
200 |
250 |
300 |
350 |
400 |
450 |
500 |
|
Dose (mL) |
1.5 |
2.0 |
3.0 |
4.0 |
5.0 |
6.0 |
7.0 |
8.0 |
9.0 |
10.0 |
● Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
Administration
Tauramox™ Injectable should be administered by subcutaneous injection under the loose skin in front of or behind the shoulder (Figure 1). Needles 1/2 to 3/4 inch in length and 16 to 18 gauge are recommended for subcutaneous injections. Use sterile, dry equipment and aseptic procedures when withdrawing and administering Tauramox™.
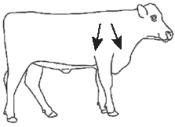
Figure 1. Sites for administration of Tauramox™ Injectable
Human Warnings
Not For Use in Humans. Keep this and all drugs out of the reach of children.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Norbrook at 1-866-591-5777. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
 |
Residue WarningsCattle must not be slaughtered for human consumption within 21 days of treatment. This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established for preruminating calves. Do not use in calves to be processed for veal. |
 |
Animal Safety Warnings
Do not use in sick, debilitated, or underweight animals. In foreign countries there have been reports of adverse effects, including death. This product should not be used in calves less than 8 weeks of age because safety testing has not been done in the U.S. in calves less than 8 weeks of age.
Environmental Warnings
Studies indicate that when moxidectin comes in contact with the soil, it readily and tightly binds to the soil and becomes inactive. Free moxidectin may adversely affect fish and certain aquatic organisms. Do not contaminate water by direct application or by improper disposal of drug containers.
OTHER WARNINGS
● Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers.
● Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance.
● Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd/flock, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method).
● A decrease in a drug’s effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
Precautions
Tauramox™ Injectable has been formulated specifically for subcutaneous injection in cattle and should not be given by other routes of administration. Subcutaneous injection can cause transient local tissue reaction that may result in trim loss of edible tissue at slaughter if animals are slaughtered within 35 days after treatment. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
Tauramox™ Injectable is effective against the migrating stage of cattle grubs (Hypoderma larvae). Treatment with Tauramox™ Injectable during the period when grubs are migrating through vital areas may cause undesirable host-parasite reactions. Killing H. lineatum when they are located in peri-esophageal tissues may cause bloat. Killing H. bovis when they are in the vertebral canal may cause staggering or hindlimb paralysis. Cattle should be treated as soon as possible after heel fly (warble fly) season to avoid this potential problem. Cattle treated with Tauramox™ Injectable at the end of fly season can be retreated during the winter without danger of grub-related reactions. Consult your veterinarian for more information regarding these secondary grub reactions and the correct time to treat with Tauramox™ Injectable.
Animal Safety
U.S. tolerance and toxicity studies have demonstrated that moxidectin injectable has an adequate margin of safety for use in cattle 8 weeks of age and older. No toxic signs were seen in growing cattle given up to 5 times the recommended dose. Calves as young as 8 weeks of age showed no toxic signs when treated with up to 3 times the recommended dose while nursing from cows concurrently treated with the recommended dose level of moxidectin injectable. Mild, transient ataxia was noted in growing cattle receiving 10 times the recommended dose and in bulls treated at 4.5 times the recommended dose. In breeding animals (bulls and cows in estrous and during early, mid and late pregnancy), treatment with at least 3 times the recommended dose had no effect on breeding performance.
Signs of toxicity include ataxia, excessive salivation, depression, and mydriasis. These signs usually occur within 12 to 48 hours post-treatment.
Storage
Store at 59° to 77°F (15° to 25°C). Exposure to temperature up to 104°F (40°C) may be tolerated provided the mean kinetic temperature does not exceed 77°F (25°C). Use within 3 months of first puncture and puncture a maximum of 56 times. If more than 56 punctures are anticipated, the use of multi-dosing equipment is recommended. If using a needle or draw-off spike larger than 16 gauge, discard any remaining product immediately after use.
Disposal
Do not contaminate water by direct application or by improper disposal of drug containers. Dispose of containers in an approved landfill or by incineration.
Package Information
Tauramox™ Injectable is available in 250 and 500 mL amber glass bottles.
Restricted Drug (CA) - Use Only As Directed
Approved by FDA under ANADA # 200-746
Tauramox™ is a trademark of Norbrook Laboratories Limited.
Manufactured by:
Norbrook Laboratories Limited, Newry, BT35 6QQ, Co. Down, Northern Ireland
Revised February 2023
015670I01
CPN: 1345029.0
9733 LOIRET BLVD., LENEXA, KS, 66219
| Telephone: | 913-599-5777 | |
| Fax: | 913-599-5766 | |
| Website: | www.norbrookinc.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
