Semintra (telmisartan oral solution)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
(telmisartan oral solution)
10 mg/mL
For oral use in cats only
Angiotensin II Receptor Blocker
Semintra (telmisartan oral solution) Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
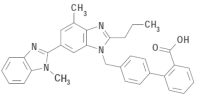
SEMINTRA (telmisartan oral solution) is a clear, colorless to yellowish viscous solution containing 10 mg/mL telmisartan. Telmisartan is an orally active, non-peptide, selective angiotensin II subtype 1 (AT1) receptor blocker. The chemical name of telmisartan is 4’ - [(1,4’ - dimethyl - 2’propyl[2,6’ - bi - 1H - benzimidazol] - 1’ - yl)methyl] - [1,1’ - biphenyl] - 2 - carboxylic acid. Its empirical formula is C33H30N4O2, its molecular weight is 514.63, and its structural formula is:
Indication: SEMINTRA is indicated for the control of systemic hypertension in cats.
Dosage and Administration
Always provide the Client Information Sheet with each prescription.The initial dose of SEMINTRA is 1.5 mg/kg (0.68 mg/lb) orally twice daily for 14 days, followed by 2 mg/kg (0.91 mg/lb) orally once daily. The dose may be reduced by 0.5 mg/kg (0.23 mg/lb) increments to a minimum of 0.5 mg/kg (0.23 mg/lb) orally once daily to manage SEMINTRA-induced hypotension. SEMINTRA can be administered directly into the mouth, or next to or on top of a small amount of food. Do not mix into food.
SEMINTRA should be administered using the dosing syringe provided in the package. The dosing syringe fits onto the bottle and has 0.1 mL incremental marks. The dose should be rounded to the nearest 0.1 mL. After administration close the bottle tightly with the cap. Rinse the dosing syringe with water and let air dry.
If the cat vomits within 30 minutes of dosing, the cat may be re-dosed.
Information for Cat Owners
Always provide the Client Information Sheet with each prescription and review it with the cat owner. Advise cat owners that adverse reactions can occur with use of SEMINTRA. The most common adverse reactions reported during the field studies included vomiting, diarrhea, lethargy, weight loss, anemia and dehydration.
Contraindications
Do not use in cats with a hypersensitivity to telmisartan.
Human Warnings: Not for human use. Keep out of reach of children.
SEMINTRA is an angiotensin II antagonist/angiotensin receptor blocker (ARB). Pregnant women should avoid contact with SEMINTRA because substances that act on the renin-angiotensin-aldosterone system (RAAS) such as angiotensin receptor blockers (ARBs) can cause fetal and neonatal morbidity and death during pregnancy in humans.
Precautions
SEMINTRA has not been evaluated in cats with systolic blood pressure >200 mm Hg.SEMINTRA can cause mild anemia or non-regenerative anemia. Cats should be monitored for anemia when initiating treatment with SEMINTRA.
SEMINTRA may cause inappetence and weight loss in some cats. Cats should be monitored for weight loss when initiating treatment with SEMINTRA. Use with caution in cats with a history of vomiting, inappetence or weight loss.
The safe use of SEMINTRA in cats with hepatic disease has not been evaluated. SEMINTRA is metabolized by the liver.
The safe use of SEMINTRA has not been evaluated in cats less than 9 months of age.
The safe use of SEMINTRA has not been evaluated in cats that are pregnant, lactating, or intended for breeding. See Human Warnings.
The safe use with other anti-hypertensive medications has not been evaluated.
Adverse Reactions
28-day Field Effectiveness Study
Safety was evaluated in a 28-day field study in 288 cats (192 SEMINTRA group cats, 96 control group cats) that received at least one dose of study drug. The control product was a vehicle control without telmisartan. Cats enrolled in the study had a median age of 14 years (7-20 years), and weighed 1.93-11.4 kg. SEMINTRA was administered orally at 1.5 mg/kg twice daily for 14 days, then 2 mg/kg once daily until study end; the control was administered at a volume equivalent to SEMINTRA. One hundred fourteen (59.4%) SEMINTRA group cats and 42 (43.8%) control group cats had at least one adverse reaction. Adverse reactions that occurred in at least 5% of either treatment group are presented in Table 1 below.
Table 1 Adverse Reactions in the 28-Day Field Study a
|
Clinical Sign |
SEMINTRA |
Control |
|
Vomiting |
46 (24.0%) |
14 (14.6%) |
|
Diarrhea |
18 (9.4%) |
4 (4.2%) |
|
Lethargy |
13 (6.8%) |
3 (3.1%) |
|
Weight loss |
13 (6.8%) |
5 (5.2%) |
|
Decreased appetite/inappetence |
13 (6.8%) |
7 (7.3%) |
|
Non-regenerative anemia |
11 (5.7%) |
2 (2.1%) |
|
Dehydration |
10 (5.2%) |
4 (4.2%) |
|
Retinal lesions (target organ damage) |
4 (2.1%) |
6 (6.3%) |
Additional adverse reactions that occurred in <5% of the SEMINTRA group included (in order of decreasing frequency): anorexia, gagging, arrhythmia, cough, heart murmur, and regenerative anemia. Additional adverse reactions representing 2-5% of the control group included azotemia, not drinking and renal failure.
Seven cats (five SEMINTRA and two control) either died or were euthanized during the study. None of the SEMINTRA group deaths were considered related to treatment.
5-month Field Effectiveness And Safety Study
The long-term safety of SEMINTRA was evaluated in an open label, 5 month field effectiveness and safety study in 107 cats that received at least one dose of SEMINTRA. Cats enrolled in the study had a mean age of 14.1 years (7-20 years) and weighed 1.92-11.4 kg. SEMINTRA was administered orally at 2 mg/kg once daily. Ninety-four cats (87.9%) had at least one adverse reaction during the study. Adverse reactions that occurred in at least 5% of cats are presented in Table 2 below.
Table 2 Adverse Reactions in the 5-Month Studya
|
Clinical Sign |
SEMINTRA |
|
Weight loss |
37 (34.6%) |
|
Vomiting |
32 (29.9%) |
|
Dehydration |
18 (16.8%) |
|
Non-regenerative anemia |
17 (15.8%) |
|
Anorexia |
14 (13.1%) |
|
Diarrhea |
12 (11.2%) |
|
Lethargy |
12 (11.2%) |
|
Decreased appetite/inappetence |
11 (10.3%) |
|
Heart murmur |
10 (9.3%) |
|
Death, Euthanasia, Found dead |
9 (8.4%) |
|
Cough |
8 (7.5%) |
|
Retinal lesions (target organ damage) |
6 (5.6%) |
Adverse reactions representing <5% of the study population were (in order of decreasing frequency): elevated liver enzymes, renal failure, tachycardia, arrhythmia, azotemia, depression, loose stool, constipation, gagging, hypotension, regenerative anemia, renal insufficiency, and vocalization.
Nine cats died or were euthanized during the study. Three cats had progressive chronic kidney disease that may have been affected by telmisartan treatment, concurrent disease, or inadequate control of hypertension. The other six cats died of causes unrelated to treatment (e.g. neoplasia).
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Boehringer Ingelheim Vetmedica, Inc. at 888-637-4251. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or at http://www.fda.gov/reportanimalae.
Clinical Pharmacology
Telmisartan is a selective angiotensin II subtype AT1 receptor blocker, with no relevant affinity for other receptors in general receptor-binding assays. Telmisartan is metabolized to the 1-0-acylglucuronide of telmisartan, which, in cats treated for 6 days at 1 mg/kg, was shown to be present in the plasma at levels approximately 21% of that of unchanged parent compound.Following an oral dose of 1 mg/kg telmisartan once daily for five days, the time to reach mean peak plasma concentration (Tmax) was 21 minutes and 32 minutes for fasted and fed cats, respectively. There was a higher systemic exposure to telmisartan in the fasted cats based on the maximum concentration (Cmax) and area under the concentration vs time curve (AUC). The mean terminal elimination half-life was approximately 8 hours. The mean systemic exposure of telmisartan (Cmax and AUC) was approximately 60% lower for female cats compared to male cats. However, dose adjustment for female cats is not necessary. An increase in dose from 1 to 5 mg/kg once daily resulted in a greater than proportional increase in telmisartan exposure. There could be low to moderate accumulation of drug upon repeated once daily or twice daily administrations of 1.5-2 mg/kg.
Effectiveness
Effectiveness was demonstrated in a 28-day multi-center, controlled, randomized and masked field study in client-owned cats with hypertension, and in an open-label 5-month field study.28-day Field Study
In the 28-day study, 288 cats with hypertension (systolic blood pressure [SBP] 160-200 mmHg) were enrolled in the study and randomized to treatment with SEMINTRA (telmisartan oral solution) (n=192) or vehicle control (n=96).
The study population included cats with hypertension associated with chronic kidney disease or controlled hyperthyroidism, or idiopathic hypertension. The per protocol population for effectiveness was 141 SEMINTRA treated cats and 79 control cats. SEMINTRA was administered orally at 1.5 mg/kg twice daily for 14 days, then 2 mg/kg once daily until study end; the vehicle control was administered at a mL/kg volume equivalent to SEMINTRA. The two primary variables for effectiveness were comparison of the SEMINTRA and control group mean SBP (mSBP) from baseline to Day 14, and a decrease in mSBP >20 mmHg in the SEMINTRA group from baseline to Day 28. Cats with SBP >180 mmHg at Days 14 or 28 were rescued and removed from the study. There was a statistically significant difference between the mSBP for the SEMINTRA group compared to the control group at Day 14 (p=0.0005). At Day 14 the SEMINTRA group mSBP decreased by 23.2 mmHg, and the control group mSBP decreased by 7.3 mmHg. At Day 28, the SEMINTRA group mSBP decreased 23.9 mmHg compared to baseline.
5-month Field Study
One hundred-seven cats from the SEMINTRA group that had successfully completed the 28-day study were enrolled in a 5-month open label study. At the beginning of the 5-month study most cats were administered SEMINTRA at 2 mg/kg once daily. Cats that experienced hypotension (defined as SBP <120 mmHg) at 2 mg/kg once daily could have the SEMINTRA dose reduced to 1 mg/kg once daily. Cats that experienced hypotension at 1 mg/kg once daily could have the SEMINTRA dose reduced again to 0.5 mg/kg once daily. Cats were evaluated for SBP, target organ damage (TOD; primarily assessed by retinal photographs), clinical pathology and adverse reactions. SBP was measured on Days 28, 56, 98, 140 and 182 and retinal photographs and clinical pathology were collected on Days 28, 98 and 182. Seventy-three (68.2%) cats completed the study (Day 182), 8 cats were removed for hypertension (SBP >180 mmHg), 2 cats were removed for hypotension, 10 cats were removed by the owner or for owner noncompliance, 8 cats were removed for new or worsening TOD, and 6 cats were removed for adverse reactions unrelated to TOD. Twenty-six cats had dose reductions to 1 mg/kg once daily to manage hypotension. Of these 26 cats, 10 had an additional dose reduction to 0.5 mg/kg once daily.
Table 3 Distribution of Cats by SBP Range and Study Day in the 5-Month Studya
|
SBP Range (mm Hg) |
Basline |
Day 28 |
Day 56 |
Day 98 |
Day 140 |
Day 182 |
|
≤ 150 |
0 (0%) |
58 (54.2%) |
55 (53.9%) |
61 (67%) |
44 (55%) |
41 (59.4%) |
|
>150-160 |
4 (3.7%) |
19 (17.8%) |
19 (18.6%) |
17 (18.7%) |
21 (26.2%) |
16 (23.2%) |
|
>160-170 |
38 (35.5%) |
18 (16.8%) |
14 (13.7%) |
10 (11%) |
12 (15%) |
7 (10.1%) |
|
>170-180 |
28 (26.2%) |
12 (11.2%) |
10 (9.8%) |
2 (2.2%) |
3 (3.8%) |
4 (5.8%) |
|
> 180 |
37(34.6%) |
0 (0%) |
4 (3.9%) |
1 (1.1%) |
0 (0%) |
1 (1.5%) |
a SBP obtained at unscheduled visits are not represented. Cats that were removed for missing >3 doses prior to SBP measurement are not included.
The most commonly used concomitant treatments during the 5-month study included (in order of frequency) antibiotics, anesthesia/sedatives/analgesia, nutritional supplements, vaccines, prescription diets, antiparasitics, thyroid treatment, antiemetics and fluid therapy.
Animal Safety: In a six-month target animal safety study, healthy cats 9 to 13 months old were administered telmisartan orally, once daily at 0, 1, 3, or 5 mg/kg body weight.
Control cats (0 mg/kg) received saline at a volume equal to the 5 mg/kg dose. There were eight cats per group (4 males, 4 females).
All cats survived the study, and there were no telmisartan-related effects on clinical observations, physical examination, body weight, ophthalmic examination, coagulation parameters, urinalysis, and gross necropsy.
Blood pressure was lower in the groups administered telmisartan compared to the control group. Blood pressure was lower starting at week 4 in the 1 mg/kg group, at week 2 in the 3 mg/kg group, and during the first week in the 5 mg/kg group. This is an expected pharmacologic effect of telmisartan. Food consumption in the 3 and 5 mg/kg group cats was lower than that of the control group.
Telmisartan-related effects on hematology parameters included lower red blood cell count, hemoglobin, hematocrit, and reticulocytes in the 3 and 5 mg/kg group cats. One 5 mg/kg group cat also had mild generalized depletion of the hematopoietic cells on bone marrow histology. In some 5 mg/kg group cats, decreases in red blood cell precursors on bone marrow cytology were considered telmisartan-related.
Blood urea nitrogen (BUN) was statistically significantly higher for the 3 mg/kg group at weeks 12 and 16, and for the 5 mg/kg group at weeks 2, 4, 7, 12, 16, 20, and 25, when compared to the control group. There were no clinical signs associated with the changes in BUN.
There was a telmisartan-related effect on lower heart weight in the 3 and 5 mg/kg groups compared to the control group, but the histopathology was normal in all treated cats. On kidney histology, there was minimal to mild hypertrophy of the juxtaglomerular apparatus in the 1, 3, and 5 mg/kg group cats. Kidney histology was normal in control group cats.
Storage Conditions: Store at or below 25°C (77°F) with excursions permitted up to 40°C (104°F). Once the bottle is opened, use the contents within six months.
How Supplied
SEMINTRA (telmisartan oral solution), 10 mg/mL, 35 mL fill volume, is supplied in a 45 mL plastic bottle with a dosing syringe.NDC 0010-4492-01
Approved by FDA under NADA # 141-501
Manufactured for: Boehringer Ingelheim Animal Health USA Inc., Duluth, GA 30096
Made in Spain
SEMINTRA is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
© 2019 Boehringer Ingelheim Animal Health USA Inc.
All Rights Reserved.
Revised 11/2019
159591-002
449201-01
CPN: 1028316.2
3239 SATELLITE BLVD., BLDG 500, DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Customer Service: | 888-637-4251 | |
| Technical Service: | 888-637-4251 | |
| Fax: | 816-236-2717 | |
| Website: | www.boehringer-ingelheim.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
