Pributazone Boluses
This treatment applies to the following species: Company: First Priority
Company: First Priority
Priority Care® 1
Phenylbutazone Boluses
For Oral Use in Horses Only
Non-steroidal anti-inflammatory drug (NSAID)
INGREDIENTS: Each bolus contains:
|
Phenylbutazone, U.S.P. |
1 gram |
Plus excipients.
DESCRIPTION & PHARMACOLOGY:
Phenylbutazone chemically is 4-butyl-1, 2-diphenyl-3, 5-pyrazolidinedione. It has the following structural formula:
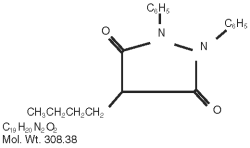
Phenylbutazone was first synthesized in 1948 and introduced into human medicine in 1949, Kuzell1,2,3, Payne4, Fleming5, and Denko6, demonstrated the clinical effectiveness of phenylbutazone in gout, gouty arthritis, acute arthritis, acute rheumatism and various other rheumatoid disorders in humans. Fabre7, Domenjoz8, Wilhelmi9, and Yourish10, have established the anti-rheumatic and anti-inflammatory activity of phenylbutazone. It is entirely unrelated to the steroid hormones.
Toxicity of phenylbutazone has been investigated in rats and mice11, Ogilvie and Sutter12, have also made a study on the chronic toxicity of phenylbutazone in dogs. They have shown that dogs receiving 10 mg. and 100 mg. per Kg. body weight, per day for 90 days, maintain good appetites, excrete normal feces, gain weight and maintain a normal blood picture. They also report no abnormal macroscopic or microscopic changes in sacrificed animals which could have been attributed to the drug.
Phenylbutazone has been used by Camberos13 in thoroughbred horses. Favorable results were reported in cases of traumatism, muscle rupture, strains and inflammations of the third phalanx. Results were not as favorable in the periodic treatment of osteo-arthritis of medial and distal bones of the hock, arthritis of the stifle and hip, arthrosis of the trapezious muscles, and generalized arthritis. Sutter14 reported a favorable response in chronic equine arthritis of long duration, fair results in a severely bruised mare, and poor results in two cases where the condition was limited to the third phalanx.
Pributazone Boluses Indications
Phenylbutazone is for the relief of inflammatory conditions associated with the musculoskeletal system in horses.
DOSAGE & ADMINISTRATION: For horses only. HORSES: Orally - 1 to 2 boluses per 500 lb. body weight. Do not exceed 4 grams daily. Reduce dosage as symptoms regress. Intermittent treatment given only when symptoms appear may be indicated.
Contraindications
Use with caution in patients who have a history of drug allergy.
PRECAUTION: Concomitant use with other antiinflammatory drugs, such as NSAIDs or corticosteroids, should be avoided or closely monitored. In the treatment of inflammatory conditions associated with infections, specific anti-infective therapy should be used concurrently.
Warning
Do not use in horses intended for human consumption.Pributazone Boluses Caution
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian. Federal (U.S.A.) law prohibits the extra-label use of this product in female dairy cattle 20 months of age or older.
AVAILABILITY: Available in 1 gm. boluses - 100 boluses per bottle.
REFERENCES
1. Kuzell, W.C., Schafferzick, R.W., Naughler, W.E., Gandia, G. and Mankle, E.A.: A.M.A. Arch. Inst. Med., 92; 646 (1953).
2. Kuzell, W.C., Schafferzick, R.W., Brown, B. and Mankle, E.A.: J.A.M.A. 149; 729 (1952).
3. Kuzell, W.C., and Schafferzick, R.W.: Calif. Med. 77; 319 (1952).
4. Payne, R.W., Shelter, M.R., Farr, C.H., Hellbaum, A.A. and Ishmall, W.K.: J. Lab. Clin. Med., 45; 331 (1955).
5. Fleming, J., and Will, G.: Ann. Rheumat. Dist., 12; 95 (1953).
6. Denko, C.W., and Rumi, D.: American Pract. 6; 1865 (1956).
7. Fabre, J., et al: Semain. Hop. (Paris) 31; 87 (1955).
8. Domenjoz, R., et al: Arzneimittel-Forsch, 5; 488 (1955).
9. Wilhelmi, G. and Pulver, R.: Arzneimittel-Forsch, 5; 221 (1955).
10. Yourish, W., Paton, B., Brodie, B., Burns, J.: A.M.A. Arch. Ophth., 53; 264 (1955).
11. Hazelton, L.W., Tusing, T.W. and Hollana, E.G.: J. Pharmacol. Exper. Ther., 109; 387 (1953).
12. Ogilvie, F.B. and Sutter, M.D.: Vet. Med. 52; 492-4 (1957).
13. Camberos, H.R.: Rev. Med. Vet. (Buenos Aires) 38; 9 (1956).
14. Sutter, M.D.: Vet. Med., 53; 83 (Feb. 1958).
Storage
Store at controlled room temperature between 15°-30°C (59°-86°F). Keep container tightly closed when not in use.Priority Care and Pributazone are registered trademarks of First Priority, Inc.
Made in U.S.A.
Manufactured By: FIRST PRIORITY, INC., Elgin, IL U.S.A.
www.prioritycare.com
ANADA 200-433, Approved by FDA
For Animal Use Only
Keep Out of Reach of Children
|
Net Contents: |
NDC# |
Reorder No. |
|
|
100 BOLUSES |
58829-334-10 |
OM036PC |
Iss. 05-09 |
CPN: 1139111.0
1590 TODD FARM DRIVE, ELGIN, IL, 60123-1146
| Telephone: | 847-289-1600 | |
| Order Desk: | 800-650-4899 | |
| Fax: | 847-289-1223 | |
| Website: | www.prioritycare.com | |
| Email: | custsvc@prioritycare.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
