NuflorGOLD
This treatment applies to the following species: Company: Intervet/Merck Animal Health
Company: Intervet/Merck Animal Health
Product Information
(florfenicol)
Injectable Solution, An Antimicrobial
300 mg/mL
NADA 141-265, Approved by FDA.
For subcutaneous use in beef and non-lactating dairy cattle only
Not for use in female dairy cattle 20 months of age or older or in calves to be processed for veal
NuflorGOLD Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
NUFLOR GOLD™ is an injectable solution of the synthetic antibiotic florfenicol. Each milliliter of sterile NUFLOR GOLD™ contains 300 mg of florfenicol, 300 mg of 2-pyrrolidone, and triacetin qs.
INDICATION: NUFLOR GOLD™ is indicated for treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis in beef and non-lactating dairy cattle.
Dosage and Administration
NUFLOR GOLD™ should be administered once by subcutaneous injection at a dose rate of 40 mg florfenicol/kg body weight (6 mL/100 lb). Do not administer more than 15 mL at each site. The injection should be given only in the neck. Injection sites other than the neck have not been evaluated.NUFLOR GOLD™ Dosage Guide*
|
ANIMAL WEIGHT (lb) |
DOSAGE |
|
100 |
6.0 |
|
200 |
12.0 |
|
300 |
18.0 |
|
400 |
24.0 |
|
500 |
30.0 |
|
600 |
36.0 |
|
700 |
42.0 |
|
800 |
48.0 |
|
900 |
54.0 |
|
1000 |
60.0 |
* Do not administer more than 15 mL at each site.
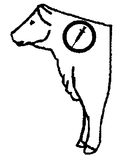
Recommended Injection Location:
Contraindications
Do not use in animals that have shown hypersensitivity to florfenicol.
WARNINGS: NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains materials that can be irritating to skin and eyes. Avoid direct contact with skin, eyes, and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information.
For customer service, to report suspected adverse reactions, or to obtain a copy of the MSDS, call 1-800-211-3573.
Precautions
Not for use in animals intended for breeding purposes. The effects of florfenicol on bovine reproductive performance, pregnancy, and lactation have not been determined. Toxicity studies in dogs, rats, and mice have associated the use of florfenicol with testicular degeneration and atrophy.Subcutaneous injection in cattle can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
 |
RESIDUE WARNINGS: Animals intended for human consumption must not be slaughtered within 44 days of treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal. |
 |
Adverse Reactions
Transient inappetence, diarrhea, decreased water consumption, and injection site swelling have been associated with the use of florfenicol in cattle. In addition, anaphylaxis and collapse have been reported post-approval with the use of another formulation of florfenicol in cattle.Clinical Pharmacology
The pharmacokinetic disposition of NUFLOR GOLD™ was evaluated in feeder calves following a single subcutaneous injection at a dose rate of 40 mg florfenicol/kg body weight. Administration of NUFLOR GOLD™ resulted in florfenicol plasma concentrations of 2 µg (mcg)/mL within two hours of injection.Table 1. Pharmacokinetic Parameter Values for Florfenicol Following a Single Subcutaneous Injection of NUFLOR GOLD™ at a Dose Rate of 40 mg Florfenicol/kg Body Weight to Feeder Calves (n=24).
|
|
Cmax |
Tmax |
AUClast |
T1/2 |
|
n |
24 |
24 |
24 |
232 |
|
Mean |
5.93 |
51 |
150 |
37.7 |
|
% CV |
38.3 |
2-121 |
20.9 |
27.3 |
Cmax: Maximum observed plasma concentration
Tmax: Time at which Cmax was observed
AUClast: Area under the plasma-concentration-time curve from time zero to the last quantifiable concentration that is equal to or greater than the limit of quantification of the validated analytical method
T1/2: Terminal elimination half-life
% CV: Percent coefficient of variance
1 Tmax is presented as the median value or range of observed values (minimum to maximum)
2 T1/2 value could not be accurately estimated for one calf
Figure 1. Mean Florfenicol Plasma Concentration versus Time Following a Single Subcutaneous Injection of NUFLOR GOLD™ at a Dose Rate of 40 mg Florfenicol/kg Body Weight in Feeder Calves (Mean ± Standard Error of the Mean)
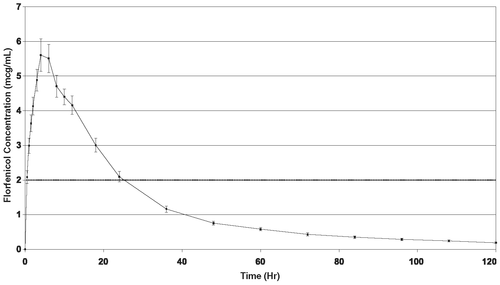
MICROBIOLOGY: Florfenicol is a synthetic, broad-spectrum antibiotic active against many Gram-negative and Gram-positive bacteria isolated from domestic animals. It acts by binding to the 50S ribosomal subunit and inhibiting bacterial protein synthesis. Florfenicol is generally considered a bacteriostatic drug, but it exhibits bactericidal activity against certain bacterial species. In vitro studies demonstrate that florfenicol is active against the BRD pathogens M. haemolytica, P. multocida, H. somni, and M. bovis and that florfenicol exhibits bactericidal activity against strains of M. haemolytica and H. somni.
The minimum inhibitory concentrations (MICs) of florfenicol were determined for non-mycoplasmal BRD isolates obtained from calves enrolled in BRD field studies in the U.S. in 2006 using methods recommended by the Clinical and Laboratory Standards Institute (M31-A2). MICs for M. bovis isolates were determined by an accepted method using Hayflick Broth with Alamar Blue (HBAN) medium under appropriate control. Isolates were obtained from pre-treatment nasal swabs from all calves enrolled at all four sites, post-treatment nasal swabs from treatment failures in the NUFLOR GOLD Injectable Solution and saline control treatment groups at three sites, and lung tissue from one calf that died in the saline control treatment group. The results are shown below in Table 2.
Table 2. Florfenicol MIC values* of indicated pathogens isolated from cattle with naturally-occurring BRD
|
Indicated pathogens |
Year of isolation |
No. of isolates |
MIC50** |
MIC90** |
MIC range |
|
Mannheimia haemolytica |
2006 |
158 |
1.0 |
1.0 |
0.5 to 32 |
|
Pasteurella multocida |
2006 |
103 |
0.5 |
0.5 |
≤ 0.125 to 16 |
|
Histophilus somni |
2006 |
85 |
≤ 0.125 |
≤ 0.125 |
≤ 0.125 to 0.25 |
|
Mycoplasma bovis |
2006 |
59 |
1.0 |
1.0 |
0.5 to 1.0 |
* The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
** The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
1 CLSI methods for M. bovis MIC determination have not been established.
The reproducibility of the method used was confirmed in 3 independent microbiology laboratories.
ANIMAL SAFETY: A target animal safety study was conducted to evaluate the effects of NUFLOR GOLD™ when administered to feeder cattle by subcutaneous injection at 1X, 3X, or 5X the labeled dose for three consecutive days (3X the labeled duration). Decreased feed consumption (inappetence), decreased water consumption, and injection site swelling were observed in the 1X, 3X, and 5X groups.
A separate injection site study conducted in cattle demonstrated that NUFLOR GOLD™ may induce a transient local reaction in the subcutaneous tissue and underlying muscle tissue.
STORAGE INFORMATION: Store between 2°-30°C (36°-86°F). Use within 28 days of first use. Refrigeration is not required. The solution is light yellow to straw colored. Color does not affect potency.
How Supplied
NUFLOR GOLD™ is packaged in 100 mL (NDC 0061-5327-01), 250 mL (NDC 0061-5327-02), and 500 mL (NDC 0061-5327-03) glass sterile multiple-dose vials.Copyright © 2009, 2011 Intervet Inc., a subsidiary of Merck & Co., Summit, NJ
All rights reserved. Made in Germany
Rev. 7/12
117634 R6 V
CPN: 1047297.2
Intervet Inc.
126 E. LINCOLN AVENUE, PO BOX 2000, Rahway, NJ, 07065
| Customer Service: | 800-521-5767 | |
| Technical Service (Companion Animal): | 800-224-5318 | |
| Technical Service (Livestock): | 800-211-3573 | |
| Website: | www.merck-animal-health-usa.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
