Kexxtone (Canada)
This treatment applies to the following species:Monensin Tablets with Controlled-Release Intraruminal Device
DIN 02451999
FOR VETERINARY USE ONLY
For oral use in dairy cattle only.
Description
Kexxtone is a controlled release formulation of monensin (as monensin sodium) contained in a plastic capsule equipped with retaining wings. Slowly releasing monensin from the opening at its end, the capsule will remain effective for an average of 95 days after administration in lactating dairy cattle.
Active Ingredient
|
monensin (as monensin sodium) |
32.4 g per capsule |
Kexxtone Indications
1. As an aid in the prevention of ketosis and subclinical ketosis (hyperketonemia) in lactating dairy cattle.1
2. For the reduction in fecal shedding of Mycobacterium avium paratuberculosis (MAP) in mature dairy cattle in high risk Johne’s disease herds as an aid in the herd control of Johne’s disease as one component of a multi-component Johne’s disease control program.2
NOTE:
1Reductions in the incidence of displaced abomasum may occur in conjunction with the reduced incidence of clinical or subclinical ketosis.
2Other considerations for effective Johne’s disease control program include: identification and culling of clinical cases and heavy shedders, and reducing the exposure of calves to the pathogen (e.g., feeding colostrum/milk to calves from animals that are disease free, using uncontaminated pasture to raise calves and replacement heifers, etc.).
Dosage and Administration
Dosage:
Kexxtone delivers an approximate average dose of 335 mg of monensin per day for approximately 95 days.
For indications 1 and 2, administer one capsule orally 2 to 4 weeks prior to expected calving date, using the Kexxtone administration tool.
NOTE: An animal safety study showed no adverse effects in dairy cows when monensin controlled release capsules were used in herds feeding Rumensin Premix up to a dose of 16 ppm monensin activity.
Administration:
Care should be exercised when administering Kexxtone to ensure the delivery to the correct location in the pharynx. Be sure that the head of the administration tool is past the base of the tongue. The correct administration tool position will be indicated by the animal beginning to swallow. Failure to do so may result in soft tissue damage or regurgitation.
THE USE OF EXCESSIVE FORCE SHOULD ALWAYS BE AVOIDED WHEN DOSING TO AVOID INJURY TO THE ANIMAL. FOLLOW DIRECTIONS CAREFULLY.
Adequate animal restraint is required to properly administer this capsule. A headgate or chute used to restrain the animal must limit forward/backward motion and allow the animal’s head to be held in the forward extended position and without pressure on the neck (to prevent choking).
Each capsule has an individual number located along the capsule body. This should be recorded with the corresponding animal identification number so that, should a capsule be regurgitated, the animal can be identified.
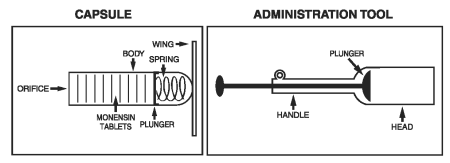
1. Identify each animal as it is dosed and match the animal identification number with the number on the side of the capsule to be used.
2. Fold wings down along capsule body and place the capsule in the head of the Kexxtone capsule administration tool, orifice end first.
3. Standing to one side of the animal, restrain it with its head and neck stretched forward and held firmly against your side. Grasp the animal with one hand in the corner of the animal’s mouth. Introduce the head of the administration tool containing the capsule into the animal’s mouth, avoiding the front teeth.
IMPORTANT: DO NOT USE EXCESSIVE FORCE IN ORDER TO AVOID TRAUMA AND DAMAGE TO THE PHARYNX AND ESOPHAGUS.
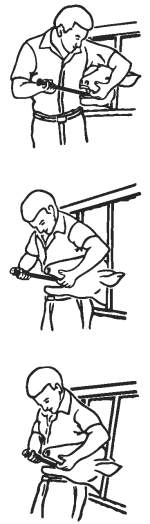
4. Once the administration tool is inside the animal’s mouth, straighten it so as to avoid the molar teeth.
5. Insert the administration tool past the base of the tongue making sure to avoid the molar teeth. As the animal swallows, the tongue will come forward and the administration tool will move easily over the base of the tongue. Do not use excessive force. Should resistance be encountered, withdraw the administration tool slightly and repeat the procedure.
6. Be sure that the head of the administration tool is past the base of the tongue. The correct administration tool position will be indicated by the animal commencing to swallow. When this occurs, eject the capsule from the administration tool by pressing the plunger.
7. Remove the administration tool from the animal’s mouth.
8. Hold treated cattle in a confined area for at least one hour after administration of the capsule to observe for failure to swallow or for regurgitation. If a regurgitated device is found, identify the animal by matching the animal identification number with the number on the capsule and redose the animal with an undamaged capsule.
9. Recheck all cattle for up to 4 days after dosing for capsule lodging in esophagus (See Cautions).
Contraindications
Do not use in cattle weighing less than 300 kg (660 lb) body weight.
CAUTIONS:
Recheck all cattle for up to 4 days after dosing for capsule lodging in the esophagus. Immediate signs of lodging within the first hours of administration include bloat which may be followed by coughing, drooling, inappetance and unthriftiness. If lodging is suspected, contact a veterinarian immediately.
Overdose:
Accidental administration of more than one intraruminal device could result in some adverse reactions which are typical of monensin overdose, including decreased appetite, scouring and lethargy. These are generally transient. The highest tolerated dose is typically between 1 mg and 2 mg monensin/kg body weight/day.
Do not allow canines, horses, other equines or guinea fowl access to formulations containing monensin. Consumption of capsule contents can be fatal in these species.
Treatment with Kexxtone has no impact on the cure of Johne’s disease, and may have no impact on decreasing the risk of culling in animals diagnosed with Johne’s disease. Treatment with Kexxtone is not an alternative to identifying and culling of clinical cases/heavy shedders for effective control of Johne’s disease.
Warnings
Do not administer Kexxtone to dairy cows in herds feeding Rumensin Premix at a level exceeding 16 ppm of monensin activity in the complete diet. No preslaughter withdrawal period or milk withholding time is required when this drug product is used according to the label directions. KEEP OUT OF REACH OF CHILDREN.
Adverse Reactions
Adverse events occur rarely with the use of this product and are most commonly reported as monensin toxicity and events related to traumatic administration of the capsule. Monensin toxicosis can occur as a result of damaged capsules and discharging of the entire contents into the rumen. Clinical signs of monensin toxicosis in cattle are diarrhea, appetite loss, and lethargy. Very rarely, monensin toxicosis can also result in heart failure and death of the animal. While reported very rarely, adverse events associated with administration of the bolus often involve trauma to the oropharynx or lodgement of the capsule in the esophagus or trachea. Accidental exposure of dogs to monensin can occur when dogs chew on regurgitated boluses. Monensin is toxic to dogs and veterinary attention should be sought if there is a suspected exposure.
Storage
Store in original container in a dry place below 30°C.Kexxtone, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
Elanco Canada Limited, 150 Research Lane, Suite 120, Guelph, Ontario N1G 4T2
|
NET: |
|
|
|
40 CAPSULES |
AH0944 |
03Jul2019 |
CPN: 1231125.1
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
