CIDR 1380 (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
progesterone releasing intravaginal insert
Veterinary Use Only
DIN 02307855
Description
CIDR® 1380 is an intravaginal progesterone releasing insert containing the naturally occurring hormone progesterone. Attached to each CIDR 1380 intravaginal insert is a polyester tail. Each CIDR 1380 intravaginal insert contains 1.38 grams progesterone EP, in molded silicone over a flexible nylon spine. The empirical formula for progesterone is C21H30O2.
The CIDR 1380 intravaginal insert provides an exogenous source of the hormone progesterone during the 7 day administration period. Removal of the CIDR 1380 intravaginal insert on treatment day 7 results in a rapid fall of plasma progesterone levels which results in synchronization of estrus in those animals responding to treatment.
Active Ingredient
Progesterone EP, 1.38 grams per CIDR 1380 intravaginal insert.
CIDR 1380 Indications
CIDR 1380 intravaginal insert is indicated for the synchronization of estrus in lactating dairy cows, suckled beef cows, and replacement beef and dairy heifers; for induction of estrous cycles in anestrous lactating dairy cows; for the advancement of first postpartum estrus in suckled beef cows; and for the advancement of first pubertal estrus in replacement beef heifers.
Dosage and Administration
Avoid contact with skin by wearing latex gloves when handling CIDR 1380 intravaginal insert.
For use with the CIDR 1380 applicator.
Lactating dairy cows: For synchronization of estrus in lactating dairy cows: Administer one CIDR 1380 intravaginal insert into the vagina of each animal to be treated for 7 days (for example, if administered on a Monday remove on the following Monday). Inject 5 mL of LUTALYSE® sterile solution (equivalent to 5 mg/mL dinoprost) on day 7, at the time of CIDR intravaginal insert removal. Observe animals for signs of estrus on days 2 to 5 after removal of the CIDR 1380 intravaginal insert and inseminate animals found in estrus following normal herd practices.
For induction of estrous cycles in anestrous lactating dairy cows: After determining that a cow is anestrous based on i) a lack of observed estrus from calving until at least 42 days in milk, ii) a lack of corpus luteum on either ovary as diagnosed by ultrasonography on two occasions at a 7 to 14 day interval (ie. Day 35 and Day 42 post-calving) OR iii) two low blood or milk progesterone concentrations, as measured at a 7 to 14 day interval (ie. Samples collected on Day 35 and Day 42), administer one CIDR 1380 intravaginal insert per anestrous cow and remove 7 days later (for example, if administered on a Monday remove on the following Monday). If insemination is intended, observe cows on days 2 to 5 after removal of the CIDR 1380 intravaginal insert and inseminate animals found in estrus following normal herd practices.
Suckled beef cows, replacement beef and dairy heifer: Administer one CIDR 1380 intravaginal insert into the vagina of each animal to be treated for 7 days (for example, if administered on a Monday remove on the following Monday). Inject 5 mL of LUTALYSE sterile solution (equivalent to 5 mg/mL dinoprost) 1 day prior to CIDR intravaginal insert removal, on day 6 of the 7 day administration period. Observe animals for signs of estrus on days 1 to 3 after removal of the CIDR 1380 intravaginal insert and inseminate animals about 12 hours after onset of estrus.
Note:
When used for the induction of estrous cycles in anestrous lactating dairy cattle, reduced conception rates were seen to the first insemination immediately following removal of the CIDR 1380 intravaginal insert compared to untreated animals. There was no difference in pregnancy rates between treated and untreated groups.
The expected retention rate is 94.8% for lactating dairy cows, 96% for suckled beef cows and 90% for beef and dairy heifers.
Insertion:
1. Restrain cattle appropriately (head catch, squeeze chute, gate, etc.) prior to administration.
2. Wash the CIDR 1380 applicator in a non-irritating antiseptic solution, and then lubricate the front portion of the CIDR 1380 applicator with a veterinary obstetrical lubricant. Only use the CIDR 1380 applicator for administration. The CIDR 1380 applicator should be washed and/or disinfected between animals.
3. Push the flexible tail of the CIDR 1380 intravaginal insert into the CIDR 1380 applicator taking care to assure the tail is extending upward through the slot of the CIDR 1380 applicator and is pointed toward the handle.
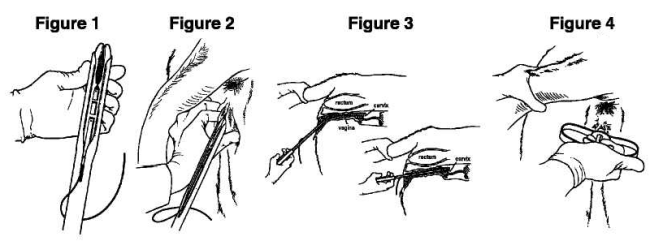
4. Fold the wings of the CIDR 1380 intravaginal insert to make it longer and continue to advance the CIDR 1380 intravaginal insert into the applicator until it is fully seated. When fully seated only the tips of the wings should protrude (one half inch) from the end of the CIDR 1380 applicator (see Figure 1 below).
5. Lubricate the protruding tips of the wings of the CIDR 1380 intravaginal insert with veterinary obstetrical lubricant.
6. Lift the tail of the animal and clean the exterior of the vulva.
7. Open the lips of the vulva and gently place the loaded CIDR 1380 applicator through the vulva. The slot in the CIDR 1380 applicator should face upwards (see Figure 2 below).
8. Once the loaded CIDR 1380 applicator is past the vulva slope the CIDR 1380 applicator slightly upwards (35 - 45° angle) by lowering the handle, and then forward, without forcing, until the CIDR 1380 applicator is fully inserted or resistance is felt (see Figure 3 below).
9. Squeeze the finger grips within the handle of the CIDR 1380 applicator to deposit the CIDR 1380 intravaginal insert in the anterior vagina (see Figure 4 below) and then pull the CIDR 1380 applicator backwards to remove it from the vagina.
10. With the CIDR 1380 intravaginal insert correctly placed, with the wings open in the anterior portion of the vagina, the tail of the CIDR 1380 intravaginal insert should be visible, pointing downward from the vulva of the animal. Tails of CIDR 1380 intravaginal inserts that protrude more than 6 cm (2.5 inches) from the vulva may be clipped to minimize removal by other animals.
Removal:
1. Remove CIDR 1380 intravaginal inserts by pulling, gently but firmly, on the protruding polyester tail.
2. CIDR 1380 intravaginal inserts have been reported to reverse direction within the vagina; therefore, if the polyester tail of the insert is not visible on the day of removal, check the vagina to determine if an insert is present.
3. Used (removed) CIDR 1380 intravaginal inserts should be stored in a sealable container until properly disposed in accordance with any applicable local or provincial regulations.
Contraindications
Do not use in animals with abnormal, immature or infected reproductive tracts.
Do not use in beef or dairy heifers of insufficient size or age for breeding.
CAUTIONS:
Do not use an insert more than once. To prevent the potential transmission of venereal and blood borne diseases the CIDR 1380 intravaginal insert should be disposed of after a single use. Increased loss of CIDR 1380 intravaginal inserts has been noted in animals housed under crowded conditions, especially in heifers. Avoid crowded conditions during treatment as other cattle, particularly heifers, may remove CIDR 1380 intravaginal inserts by pulling on the tail of the CIDR 1380 intravaginal insert. If loss rates are high re-evaluate insertion technique and cattle handling facilities. Safety of CIDR 1380 intravaginal insert has not been demonstrated in beef cows that are less than 20 days postpartum or in lactating dairy cows that are less than 40 days postpartum. Contact a veterinarian if abnormal bloody discharge is observed during treatment. Care must be taken when using the CIDR 1380 applicator so as not to damage the vagina. Animals in poor condition resulting from illness or nutritional stress may not respond to this drug.
Warnings
No milk withholding time is required when the CIDR 1380 intravaginal insert is used according to the label. Treated animals must not be slaughtered for use as food for at least 1 day after removal of the CIDR 1380 intravaginal insert.
Warning for the person administering CIDR 1380 intravaginal inserts:
Progesterone is a potent steroid hormone and may cause adverse effects on the reproductive system in cases of high or prolonged exposure.
Pregnant women should avoid using CIDR 1380 intravaginal inserts.
The product may cause skin and eye irritation, as well as allergic skin rashes.
Wear latex gloves when handling CIDR 1380 intravaginal inserts; insert the device using the
applicator.
Wash hands and exposed skin with soap and water after use.
Avoid release to the environment.
Keep out of the reach of children.
Adverse Reactions
The following may be noticed: Clear, cloudy or bloody mucus on the outside of CIDR 1380 intravaginal insert when removed from animals. The mucus may have an offensive odour. This is a result of mild irritation to the vaginal lining by the presence of the CIDR 1380 intravaginal insert, and generally clears between the time of removal and insemination. Such irritation does not affect fertility.Storage
Store below 30°C.PRESENTATION: CIDR 1380 intravaginal insert is available in bags of 10 inserts.
Zoetis®, CIDR and Lutalyse are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
39006610-02/23
1421-09-5
March 2023
CPN: 1198351.6
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
