Triderma Cream Prescribing Information
Package insert / product label
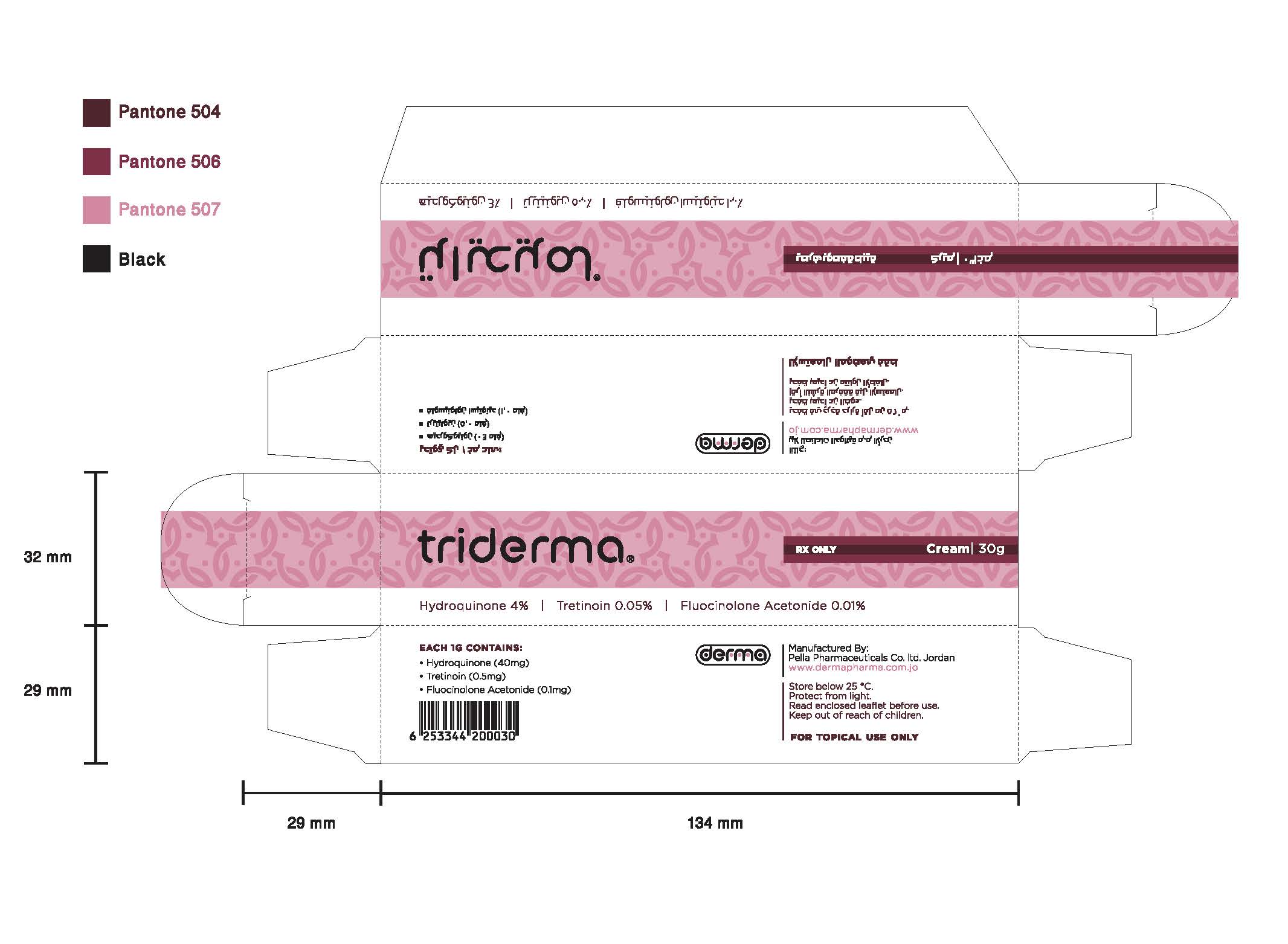
Generic name: hydroquinone, tretinoin, fluocinolone acetonide
Dosage form: cream
Drug class: Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on May 29, 2024.
On This Page
Composition
Each 1 g contains: Hydroquinone 40 mg, Tretinoin 0.5 mg and Fluocinolone Acetonide 0.1 mg.
Excipients: Emulsifying Wax, Cetyl Alcohol, Glycerin, Cetostaeryl Alcohol, Glycerol Monostearate, Stearic Acid, White Soft Paraffin, Sodium Metabisulfite, Methylparaben, Citric Acid, Butylated Hydroxyanisole, Propylparaben, and Purified Water.
Triderma Cream Description
Fluocinolone acetonide is a synthetic fluorinated corticosteroid for topical dermatological use and is classified therapeutically as an anti-inflammatory. It is a white crystalline powder that is odorless and stable in light. Hydroquinone is classified therapeutically as a de-pigmenting agent. It is prepared from the reduction of p-benzoquinone with sodium bisulfite. It occurs as fine white needles that darken on exposure to air. Tretinoin is all-trans-retinoic acid formed from the oxidation of the aldehyde group of retinene to a carboxyl group. It occurs as yellow to light-orange crystals or crystalline powder with a characteristic odor of ensilage. It is highly reactive to light and moisture. Tretinoin is classified therapeutically as a keratolytic.
Indications and Usage for Triderma Cream
Triderma is indicated for the short-term intermittent treatment of moderate to severe melasma of the face, in the presence of measures for sun avoidance, including the use of sunscreens.
Contraindications
Triderma is contraindicated in individuals with a history of hypersensitivity, allergy, or intolerance to this product or any of its components.
Precautions
Triderma contains hydroquinone and tretinoin that may cause mild to moderate irritation. Local irritation, such as skin reddening, peeling, mild burning sensation, dryness, and pruritus may be expected at the site of application. Transient skin reddening or mild burning sensation does not preclude treatment. If a reaction suggests hypersensitivity or chemical irritation, the use of the medication should be discontinued.
Triderma also contains the corticosteroid fluocinolone acetonide. Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced by systemic absorption of topical corticosteroid while on treatment. If HPA axis suppression is noted, the use of Tridema should be discontinued. Recovery of HPA axis function generally occurs upon discontinuation of topical corticosteroids.
Pregnancy
Teratogenic Effects: Pregnancy Category C.
Triderma Cream contains the teratogen, tretinoin, which may cause embryo-fetal death, altered fetal growth, congenital malformations, and potential neurologic deficits. It is difficult to interpret the animal studies on teratogenicity with
Tridrema Cream, because the availability of the dermal applications in these studies cannot be assured, and comparison with clinical dosing is not possible.
There are no adequate and well-controlled studies in pregnant women.
Triderma should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Lactation
Corticosteroids, when systemically administered, appear in human milk. It is not known whether topical application of Triderma could result in sufficient systemic absorption to produce detectable quantities of fluocinolone acetonide, hydroquinone, or tretinoin in human milk. Because many drugs are secreted in human milk, caution should be exercised when Triderma is administered to a nursing woman. Care should be taken to avoid contact between the infant being nursed and Triderma.
Drug Interactions
Patients should avoid medicated or abrasive soaps and cleansers, soaps and cosmetics with drying effects, products with high concentration of alcohol and astringent, and other irritants or keratolytic drugs while on Tridema treatment. Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Warnings
Triderma contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and lifethreatening asthmatic episodes in susceptible people.
Triderma contains hydroquinone, which may produce exogenous ochronosis, a gradual blue-black darkening of the skin, whose occurrence should prompt discontinuation of therapy. The majority of patients developing this condition are Black, but it may also occur in Caucasians and Hispanics.
Cutaneous hypersensitivity to the active ingredients of Triderma has been reported in the literature.
In a patch test study to determine sensitization potential in 221 healthy volunteers, three volunteers developed sensitivity reactions to the product or its components.
Triderma Cream Dosage and Administration
Triderma should be applied once daily at night. It should be applied at least 30 minutes before bedtime.
Gently wash the fuce and neck with a mild cleanser. Rinse and pat the skin dry. Apply a thin film of the cream to the hyper pigmented areas of melasma including about 1/2 inch of normal appearing skin surrounding each lesion. Rub lightly and uniformly into the skin. Do not use occlusive dressing.
During the day, use a sunscreen of SPF 30, and wear protective clothing. Avoid sunlight exposure. Patients may use moisturizers and/or cosmetics during the day.
Duration of Action:
Triderma Cream is for short-term (up to 8 weeks) treatment of moderate to severe melasma of the face. It is not for longterm (more than 8 weeks) or maintenance (continuous) treatment of melasma. Milder forms of melasma may not need treatment with medicine. Melasma can also be managed by staying out of the sun or by stopping the use of birth control methods that involve hormones.
Adverse Reactions/Side Effects
The following local adverse reactions have been reported infrequently with topical corticosteroids. They may occur more frequently with the use of occlusive dressings, especially with higher potency corticosteroids. These reactions are listed in an approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, acneifom, eruptions, hypopigmentations, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, and miliaria.
THIS IS A MEDICAMENT
- Medicament is a product which affects your health and its consumption contrary to instructions is dangerous for you.
- Strictly follow the doctor's prescription, the method of use and the instruction of the pharmacist who sold the medicament.
- The doctor and the pharmacist are experts in medicine, its benefits and risks.
- Do not by yourself interrupt the period of treatment prescribed for you.
- Do not repeat the same prescription without consulting your doctor.
- Keep medicament out of reach of children.
| TRIDERMA
hydroquinone, tretinoin, fluocinolone acetonide cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pella Pharmaceutical Co. Ltd (562370925) |
More about Triderma (fluocinolone / hydroquinone / tretinoin topical)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical depigmenting agents